
Factors associated with non-completion of palliative radiotherapy for spinal metastasis in patients with terminal cancer: a retrospective study
Highlight box
Key findings
• The proportion of patient death was higher in the incomplete radiotherapy (RT) group than in the complete group.
• The incomplete group showed significantly higher palliative performance scale scores and lower total lymphocyte count (TLC) before palliative RT than the complete group.
What is known and what is new?
• RT, specifically fractionated irradiation, is administered to patients with terminal cancer to relieve the pain caused by spinal metastases. However, palliative RT cannot be completed in some cases.
• This study outlines the factors that may cause non-completion of palliative RT, except the single-fraction for painful spinal metastasis in patients with terminal cancer.
What is the implication, and what should change now?
• Pre-RT TLCs can predict non-completion of palliative RT in patients with terminal cancer.
• These markers can potentially serve as guides for setting the treatment duration for palliative RT in the future.
Introduction
Background
Radiotherapy (RT) is administered to patients with terminal cancer to relieve the pain caused by spinal metastases (1). Regarding the optimal dose and fractionation of RT for painful spinal metastases, radiation oncologists in Japan administer RT of 20 Gy in 5 fractions or 8 Gy in 1 fraction (2,3), in addition to 30 Gy in 10 fractions, depending on the status of the primary or metastatic lesions and the patient’s estimated prognosis and social background. For patients with expected long-term survival, fractionated irradiation is often selected (4). However, older patients with cancer are prone to deterioration of general conditions owing to age-related decline in immunity, cachexia induced by tumors, inflammation, adverse events caused by systemic anticancer treatment, and pre-existing conditions. Consequently, the estimated prognoses of older patients greatly deviate from the actual duration of their survival. Moreover, not all of the scheduled fractionated RT doses can be administered in some cases; therefore, palliative RT administered in the terminal stage of cancer is not completed.
Rationale and knowledge gap
To better tailor the recommended RT dose/fractionation schemes, radiation oncologists should accurately predict prognosis in patients with terminal cancer. Optimistic prediction of prognosis might lead to non-completion of RT. For example, when the general condition of a patient with an estimated prognosis of 1–3 months rapidly deteriorates, the subjective prediction of prognosis based on clinical experience and performance status (PS) is not always reliable. Clinicians’ predictions of survival for patients with advanced cancer are often inaccurate and overestimated (5). However, whether optimistic prediction of prognosis or other factors affect non-completion of palliative RT (not including single-fraction RT) has not been established. Therefore, we investigated prognostic factors influencing non-completion of palliative RT, which have rarely been reported to date.
Objective
This study aimed to explore the factors that may cause non-completion of palliative RT (not including single-fraction RT) for painful spinal metastasis in patients with terminal cancer. We present this article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-540/rc).
Methods
To create a diverse sample set of patients encompassing a wide distribution of diagnoses, disease status, and laboratory data, all patients treated with RT in the past 8 years were included in our observational study. This single-center retrospective study was conducted at Kawasaki Medical School Hospital, a regional cancer center in Okayama Prefecture. Patients with terminal cancer who were registered on the cancer board, dedicated to the early detection and prevention of skeletal-related events from metastatic spinal tumors between January 1, 2014, and December 31, 2021, were considered eligible for inclusion. To establish a diverse patient sample reflecting a broad spectrum of diagnoses, disease statuses, and laboratory data, we included all patients treated with RT whose data were recorded in the cancer board registry after its initiation in 2014. From this dataset, we only selected records of patients who survived for ≤3 months. Terminal cancer is often defined as a refractory disease stage with a prognosis of ≤6 months of survival (6). However, as anticancer treatment is often administered to patients with a prognosis of 6 months, the terminal stage in this study was defined as a survival period of ≤3 months.
The patients were not indicated for surgical intervention and received the best palliative RT for painful spinal metastases. However, patients who received a single-fraction of irradiation were excluded from the study, as non-completion of palliative irradiation was the main focus. The patients were not indicated for orthopedic procedures and received palliative RT (not including single-fraction RT) for painful spinal metastases. Patients who could not complete RT owing to social reasons, such as transfer to another hospital or refusal to receive RT for financial reasons, or owing to medical reasons, such as treatment discontinuation determined at an in-hospital conference with radiation oncologists certified by the Japanese Society for Radiation Oncology, were excluded from the incomplete group, as quantitative evaluation of factors that lead to the non-completion of palliative RT is difficult. Moreover, patients with hematologic tumors, patients aged <18 years, those lacking computed tomography (CT) images, and those with tumors directly infiltrating the major psoas muscle were excluded from the study. Patients with hematologic tumors were excluded because changes in their blood counts differ from those of patients with other cancers, and their common causes of death differ from those of patients with solid tumors. Patients aged <18 years were excluded because their normal ranges of hematologic test data differ from those of adults. We excluded patients lacking CT images taken 1–3 months or immediately before RT, which were used to measure the psoas muscle index (PMI) (7).
Investigation items
The day palliative RT was initiated for the included patients was set as the baseline. We retrospectively extracted the medical information of the patients from their medical records. The information extracted included hematologic test data obtained 1–3 months before and after baseline, Eastern Cooperative Oncology Group (8) PS, and palliative performance scale (PPS) scores (9) recorded at the time of CT. The data on patient characteristics included sex, age at diagnosis, spinal instability neoplastic score before RT (≥7), survival status (death within 1 week after RT or within 1 month after RT), survival period, number of irradiation fractions (≥2 to <10, 10, and >10), type of carcinoma (lung cancer or other cancers), presence or absence of other metastatic lesions, use of bone-modifying agents, and type of bone metastasis (osteolytic, mixed, osteoblastic, or trabecular). Regarding the spinal instability neoplastic score, the scores proposed by the Cancer Board for Metastatic Spinal Tumors were used. Regarding the hematologic test items analyzed in this study, the following nutritional, inflammatory, and hepatic markers and blood counts were selected with reference to those evaluated in previous studies (10-15): serum albumin (Alb), C-reactive protein, lactate dehydrogenase, and total lymphocyte count (TLC). To exclude the impact of cancer treatment on hematologic test data, we used data collected ≥2 weeks after the latest anticancer treatment (surgery, chemotherapy, hormone therapy, or immunotherapy). Since the patients’ records did not include the PPS score, the lead author retrospectively assessed the sitting and standing levels, mobility, symptoms, activities of daily living, intake, and level of consciousness [Japan Coma Scale (9,16)] of the patients and determined their PPS scores after the study initiation. The levels of necessary assistance for activities (bed mobility, transfer, cleaning of the oral cavity, eating, and dressing) were extracted from the nursing records of hospitalized patients and used as references.
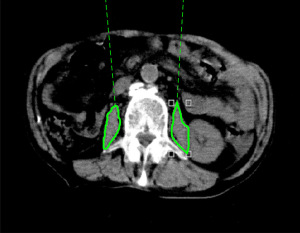
PMI was calculated using CT images taken for diagnosis 1–3 months before RT, CT images taken immediately before RT and used for positioning of irradiation sites during RT planning, and CT images taken for diagnosis 1–3 months after RT. The tracing tool (6) provided in the image viewing system of the CT device was used to calculate the cross-sectional areas of the left and right major psoas muscles at the third lumbar vertebral level. The cross-sectional area of the skeletal muscle was then divided by the height squared. The resultant skeletal muscle index was used for evaluation (Figure 1). Charlson comorbidity index (CCI) (17) and age-adjusted CCI (aCCI) (18) were divided into two categories (CCI: <7 and ≥7; aCCI: <10 and ≥10) for evaluation.
Statistical analyses
SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. We used the Shapiro-Wilk test to assess the normality of the distribution of continuous variables. Patients were broadly divided into complete and incomplete RT groups, and each investigation item was compared between the two groups using Fisher’s exact test. We used an unpaired t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Correlations between items were analyzed using Pearson’s product-moment correlation coefficient and Spearman’s rank correlation coefficient if normally distributed. Specifically, we selected risk factors for inclusion in multivariate regression using rational clinical judgment on potential confounding parameters of the association between risk factors and outcomes. Furthermore, we conducted multivariate logistic regression analysis with forced entry or forward regression by former Fisher’s exact test for risk factors that had a significant difference (a two-tailed P value <0.05 was considered statistically significant) and obtained the odds ratio and 95% confidence intervals for the risk factors to determine statistically significant associations between non-completion of palliative RT and the risk factor. For patients whose blood samples were collected multiple times within 1 week, the hematologic test data for all investigation items measured using each blood sample were counted as one event, and the mean value for each item was calculated by dividing the obtained values by the total number of events. Changes that occurred from baseline up to the time of death were explored. We considered that the missing values were random and chose a method to exclude the missing values.
Ethical considerations
This study was conducted using pre-existing materials and data from our research institution in accordance with the “Ethical Guidelines for Medical and Health Research Involving Human Subjects” (Ministry of Education, Culture, Sports, Science and Technology, 2014; partially revised in February 2017, Public Notice of the Ministry of Health, Labour and Welfare No. 3). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Kawasaki Medical School Hospital (approval numbers: 3579-02, 5054-02, and 5590-00), and the requirement of individual informed consent for this retrospective analysis was waived.
Results
Between January 1, 2014, and December 31, 2021, palliative RT for painful spinal metastasis was administered to 476 patients at our hospital. Of these, 19 patients did not complete RT (completion rate: ≥50% for 13 patients and <50% for 6 patients). After excluding patients according to the exclusion criteria, 58 patients with terminal-stage cancer (patients who died within 3 months of palliative radiation treatment) were included in the complete group and 9 patients in the incomplete group (completion rate: ≥50% for 5 patients and <50% for 4 patients). The characteristics of the patients in both groups are shown in Table 1. In the complete group, the primary tumors were gastrointestinal cancer (20 patients), lung cancer (15 patients), urological cancer (10 patients), breast cancer (4 patients), other cancers (4 patients), synchronous cancer (4 patients), and unknown (1 patient). In the incomplete group, the primary tumors were lung cancer (3 patients), gastrointestinal cancer (3 patients), urological cancer (2 patients), and breast cancer (1 patient). No patient in the incomplete group had synchronous cancer. The median survival period was 39.5 days (range, 4–91 days) in the complete group and 19 days (range, 1–45 days) in the incomplete group. In the complete group, the number of irradiation fractions was >2 to <10 in 20 patients, 10 in 34 patients, and >10 in 4 patients. In the incomplete group, the number of irradiation fractions was >2 to <10 in 2 patients, 10 in 7 patients, and >10 in 0 patient. No significant difference was noted in the number of irradiation fractions between these groups. The proportion of patients who died within 1 week and within 1 month was higher in the incomplete group than in the complete group (P=0.046 and P=0.034, respectively; Figure 2A). However, no deaths were noted during the RT period. The reasons for non-completion of RT and fraction size are outlined in Table 2. The complete group showed significantly higher TLC measured 1 week before RT (pre-TLC) than the incomplete group (P=0.013; Figure 2B), whereas the incomplete group showed significantly higher PPS scores before RT (pre-PPS) than the complete group (P=0.012; Figure 2C).
Table 1
| Prognostic factor | Complete group (n=58) | Incomplete group (n=9) | P value (Uni) | P value (Multi) | OR (95% CI) |
|---|---|---|---|---|---|
| Sex | >0.999† | – | – | ||
| Male | 41 (70.7) | 6 (66.7) | |||
| Female | 17 (29.3) | 3 (33.3) | |||
| SINS | 0.295† | – | – | ||
| <7 | 25 (43.1) | 2 (22.2) | |||
| ≥7 | 33 (56.9) | 7 (77.8) | |||
| Fraction times | 0.700§ | – | – | ||
| ≥2 to <10 | 20 (34.5) | 2 (22.2) | |||
| 10 | 34 (58.6) | 7 (77.8) | |||
| >10 | 4 (6.9) | 0 | |||
| Type of the malignant tumor | 0.693† | – | – | ||
| Lung cancer | 15 (25.9) | 3 (33.3) | |||
| Other cancer | 43 (74.1) | 6 (66.7) | |||
| Other metastatic lesions | 50 (86.2) | 6 (66.7) | 0.159† | – | – |
| Bone-modifying agent | 40 (69.0) | 6 (66.7) | >0.999† | – | – |
| Type of bone metastasis | 0.368† | – | – | ||
| Osteolysis | 39 (67.2) | 6 (66.7) | |||
| Mixture | 6 (10.3) | 2 (22.2) | |||
| Osteoblast | 9 (15.5) | 0 | |||
| Trabeculae | 4 (6.9) | 1 (11.1) | |||
| CCI | >0.999† | – | – | ||
| <7 | 26 (44.8) | 4 (44.4) | |||
| ≥7 | 32 (55.2) | 5 (55.6) | |||
| aCCI | 0.437† | – | – | ||
| <10 | 13 (22.4) | 3 (33.3) | |||
| ≥10 | 45 (77.6) | 6 (66.7) | |||
| Age (years) | 70.8±10.2 | 68.8±13.6 | 0.598‡ | – | – |
| Pre-PMI | 4.2 (3.4, 5.0) | 2.9 (2.6, 5.1) | 0.282§ | – | – |
| Post-PMI | 3.9 (3.2, 5.0) | 2.5 (2.2, 4.7) | 0.044*§ | n.e. | n.e. |
| Pre-Alb | 3.3±0.6 (n=56) | 3.4±0.6 | 0.681‡ | – | – |
| Alb | 3.1±0.6 (n=57) | 2.8±0.7 | 0.276‡ | – | – |
| Pre-CRP | 1.6 (0.5, 6.4) (n=54) | 1.9 (0.3, 3.0) (n=8) | 0.793§ | – | – |
| CRP | 3.8 (1.3, 6.5) (n=53) | 5.4 (2.5, 10.1) | 0.285§ | – | – |
| Pre-LDH | 285.0 (220.0, 359.0) (n=55) | 285.0 (225.5, 511.5) | 0.582§ | – | – |
| LDH | 313.0 (241.0, 501.0) (n=57) | 364.0 (297.0, 636.5) | 0.182§ | – | – |
| Pre-TLC | 1,080.0 (727.5, 1,410.0) (n=52) | 590.0 (331.0, 869.8) | 0.013*§ | 0.048*§ | 0.998 (0.996–1.00) |
| TLC | 870.0 (480.0, 1,310.0) (n=55) | 510.0 (321.5, 929.6) | 0.153§ | – | – |
| Pre-PS | 1.4±0.7 | 1.4±0.5 | 0.904‡ | – | – |
| PS | 1.9±1.0 | 2.4±1.0 | 0.109‡ | – | – |
| Pre-PPS | 67.8±12.4 | 78.9±7.8 | 0.012*‡ | 0.039*‡ | 1.097 (1.005–1.198) |
| PPS | 57.4±14.1 | 52.2±21.1 | 0.342‡ | – | – |
| SINS | 6.7±2.6 | 6.8±1.8 | 0.953‡ | – | – |
| CCI | 7.0 (6.0, 7.5) | 7.0 (6.0, 9.0) | 0.899§ | – | – |
| aCCI | 10.4±1.7 | 10.7±2.9 | 0.748‡ | – | – |
Data are presented as N, n (%), mean ± standard deviation and median (interquartile range). *, P value <0.05; †, Fisher’s exact test; ‡, unpaired t-test; §, Mann-Whitney U-test. The pre-XX measurements were taken >1 week before radiotherapy, the post-YY measurements were taken <1 week after radiotherapy, and the Alb, LDH, and CRP measurements were taken during radiotherapy. Uni, univariate analysis; Multi, multivariate analysis; OR, odds ratio; CI, confidence interval; SINS, spinal instability neoplastic score; CCI, Charlson comorbidity index; aCCI, age-adjusted Charlson comorbidity index; PMI, psoas muscle index; n.e., not entered; Alb, albumin; CRP, C-reactive protein; LDH, lactate dehydrogenase; TLC, total lymphocyte count; PS, performance status (Eastern Cooperative Oncology Group); PPS, palliative performance scale.
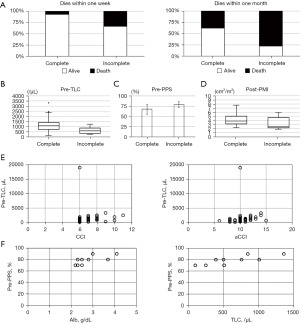
Table 2
| Patient numbers | Reasons for non-completion of RT and fraction size | Fraction times |
|---|---|---|
| No. 1 | Exacerbation of the primary tumor and heart failure | 8/10 |
| No. 2 | Exacerbation of respiratory status due to lung metastases, onset of delirium, and unrest | 3/10 |
| No. 3 | Radiation sickness (strong malaise) | 3/5 |
| No. 4 | Exacerbation of symptoms due to brain metastases | 9/10 |
| No. 5 | Exacerbation of respiratory status due to lung metastases | 1/10 |
| No. 6 | Sudden bone marrow infiltration | 5/10 |
| No. 7 | Exacerbation of respiratory status due to lung metastases | 5/10 |
| No. 8 | Exacerbation of respiratory status due to primary lung cancer | 3/5 |
| No. 9 | Exacerbation of respiratory status due to pneumonia | 6/10 |
RT, radiotherapy.
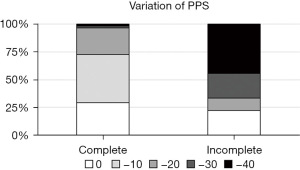
The complete group showed significantly higher PMI after RT (post-PMI) than the incomplete group (P=0.044; Figure 2D). Spearman’s correlation analysis showed that the pre-TLC of the complete group was significantly correlated with CCI (P=0.022) and aCCI (P=0.007; Figure 2E). Pearson’s and Spearman’s correlation analyses showed that the pre-PPS of the incomplete group was significantly correlated with Alb level (P=0.036) and TLC (P=0.018), respectively (Figure 2F). In multivariate analysis, pre-TLC and pre-PPS were significant prognostic factors associated with the incomplete group (P=0.048 and 0.039, respectively; Table 1). In comparison to the complete group, the incomplete group included several patients whose PPS scores rapidly decreased by 30–40 points from baseline (Figure 3).
Discussion
We investigated the predictive factors for non-completion of palliative RT for painful spinal metastases in patients with terminal cancer. We did not include patients receiving a single irradiation of 8 Gy but included patients receiving fractionated irradiation, as the fractionated irradiation allowed us to differentiate non-completion from completion of RT. In a mail survey of the Japanese population conducted by the Ministry of Health, Labour and Welfare in 2017, 50–70% of people responded that they want to stay at home if they develop terminal-stage cancer, and nearly 70% answered that they want to die at home (19). Given the results of that survey, we considered that when radiation oncologists administer palliative RT to patients with terminal cancer, setting the appropriate completion or non-completion for the prognosis of each patient is important for providing a suitable care environment according to the wishes of the patients and their families. This is consistent with the findings of Patel et al. (20), who reported that physicians should consider ceasing palliative RT earlier in patients with terminal illness with worsening PS and multiple signs of clinical deterioration, aiming at transitioning care toward comfort measures and avoiding ineffective treatment delivery in the last 4 weeks of life.
Comparison with similar researches
TEACHH (21), Chow et al. (22), and palliative home care setting (23) are prognostic models that were developed for patients with metastatic cancer being treated with palliative RT. These models may help clinicians provide quality palliative care to their patients with advanced cancer and their families. However, detailed examination items such as pre-TLC values and PMI, which were used in the present study, were not included in these models.
Patients with terminal cancer are likely to experience rapid deterioration, causing worsening of their general condition and death within 1–2 days. Such sudden changes are a possible cause of insufficient decision-making support for patients and their families, considering that the patients eventually die early (24). This sudden change is likely to prevent radiation oncologists from ultimately providing a care environment according to the wishes of patients and their families. This is because radiation oncologists tend to be optimistic in predicting prognoses (5) and thus may not prescribe irradiation fractions that are appropriate for a patient’s true prognosis, resulting in the patient being unable to complete RT.
Key findings
We retrospectively collected various data that are predictors of prognosis, such as Eastern Cooperative Oncology Group PS, PPS, PMI, CCI, and aCCI, and analyzed the changes in their values. Since biochemistry and blood count data are abnormal during the terminal stage of cancer (10), some data obtained from blood samples are used to calculate scores for predicting the prognoses of patients with terminal cancer (11,12). Low TLC (11), low Alb levels (13), high C-reactive protein levels (14), and high lactate dehydrogenase levels (15) have been associated with poor prognosis in patients with various solid cancers. In previous studies, PPS scores (9) showed a rapid decline during the 4 weeks before death (25).
Explanations of findings
Among the factors associated with poor prognosis, such as decreased continuity of cancer treatment, increased incidence of complications, and decreased overall survival rate, a decrease in muscle mass and strength, known as sarcopenia, has recently been attracting attention. PMI is used for the evaluation of sarcopenia, and it is an important marker for various malignant tumors (26-31). For radiation oncologists who plan RT and perform CT to localize the radiation site, measuring PMI on CT images is not difficult. Thus, we used PMI measured before and after RT as a predictor or reference to determine irradiation dose and fractionation in this study. Since several older patients and many patients with comorbidities were included in this study, we also used CCI and aCCI as predictors to determine irradiation dose and fractionation.
Multivariate analysis of each investigation item showed that the pre-TLC and pre-PPS of the complete and incomplete groups were significantly different. More specifically, pre-TLC tended to be higher in the complete group than in the incomplete group, suggesting the activation or maintenance of immunity in the complete group. A high pre-TLC seemed to be constant among the patients who completed RT. Moreover, contrary to our expectations, the pre-PPS was higher in the incomplete group than in the complete group. However, the complete group included more patients with good oral intake and consciousness than the incomplete group, which may have been reflected sharply in the PPS. The most likely reason for the results shown in Figure 3 is rapid and unpredictable disease progression. Organ disorders caused by the progression of the primary or metastatic lesion were considered the reason for rapid disease exacerbation in more than half of the patients in the incomplete group. The higher PPS scores observed in the incomplete group are consistent with the findings of Seow et al. (25), who reported that the PPS scores of patients with terminal cancer rapidly worsen during the 4 weeks before death. Therefore, we considered that rapid deterioration of PPS before and after treatment was the cause of incomplete irradiation. The post-PMI of the complete group was significantly higher than those of the incomplete group, reflecting undernutrition and sarcopenic frailty. Some patients showed a gradual decrease in levels of the nutritional marker Alb and the inflammatory marker C-reactive protein before the deterioration of PS and PPS. Although this decrease in Alb levels is consistent with the findings of a previous study, which indicated a gradual decrease in Alb levels during the 6 months before death (13), no significant differences were noted in other predictors and reference markers that strongly affect the determination of RT completion or non-completion, such as pre-TLC, between the two groups. This may be attributable to the fact that several patients aged ≥65 years were included in this study. Older patients have a high risk for undernutrition, sarcopenic frailty, and multiple complications owing to aging. Older patients with advanced cancer often have these conditions concurrently (32,33). Furthermore, CCI and aCCI are considered useful for predicting long-term prognosis in the fields of gastrointestinal cancer, gynecological cancer, and orthopedic surgery (17,18). However, these indices may not be suitable for investigating the predictors of short-term prognosis, as in this study. Bollen et al. (34) reported that PS is a strong prognostic predictor of survival in patients with spinal metastases, which is contrary to our study findings, as PS was not found to be a strong prognostic predictor. Furthermore, although the white blood cell count used in the nomogram created by the Skeletal Oncology Research Group should be a prognostic factor, to the best of our knowledge, no study has clarified its potential as a prognostic factor for pre-TLC.
Strengths and limitations
This study has some limitations. First, the exclusion criteria may introduce selection bias and limit the generalizability of the findings. Second, it has a small sample size. Third, this was a single-center study, and several patients aged ≥65 years were included. Thus, the findings of this study may be less generalizable than those of studies with patients from a wide range of age groups. Moreover, patients who discontinued RT owing to social reasons were excluded, and the hematologic tests or CT scans were not performed at a sufficient frequency for some patients who experienced rapid deterioration and died within 1–2 weeks after RT. The data on such patients may have been strongly reflected in the overall trend; therefore, the possibility of statistical bias cannot be ruled out. As this was a retrospective observational study, we could not control the frequency or interval of CT scans or blood sampling. However, this is the first clinical study wherein patients with terminal-stage cancer who received palliative RT (not including single-fraction RT) for painful spinal metastasis were categorized into those who completed RT and those who did not, and the changes in their hematologic test data, PS, PPS, PMI, CCI, and aCCI were compared. These results present useful predictors for determining the appropriate completion or non-completion of palliative RT for painful spinal metastasis that should be administered to patients with terminal cancer.
Conclusions
This study demonstrated that pre-TLC and rapid decrease in PPS before and after treatment are candidate predictors or reference markers for predicting non-completion of palliative RT for painful spinal metastasis in patients with terminal cancer. We believe that these markers could serve as guides for setting the number of fractions (treatment duration) for palliative RT in the future.
Acknowledgments
The authors would like to thank Statista, Co., Ltd. for useful statistical comments and Honyaku Center Inc. for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-540/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-540/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-540/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-540/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Kawasaki Medical School Hospital (approval numbers: 3579-02, 5054-02, and 5590-00), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giammalva GR, Ferini G, Torregrossa F, et al. The Palliative Care in the Metastatic Spinal Tumors. A Systematic Review on the Radiotherapy and Surgical Perspective. Life (Basel) 2022;12:571. [Crossref] [PubMed]
- Rades D, Segedin B, Schild SE, et al. Identifying patients with malignant spinal cord compression (MSCC) near end of life who can benefit from palliative radiotherapy. Radiat Oncol 2022;17:143. [Crossref] [PubMed]
- Nieder C, Haukland EC, Mannsåker B. Shortened Palliative Radiotherapy Results in a Lower Rate of Treatment During the Last Month of Life. Cureus 2022;14:e21617. [Crossref] [PubMed]
- Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:4-12. [Crossref] [PubMed]
- Cheon S, Agarwal A, Popovic M, et al. The accuracy of clinicians’ predictions of survival in advanced cancer: a review. Ann Palliat Med 2016;5:22-9. [PubMed]
- Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med 1996;335:172-8. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200-5. [Crossref] [PubMed]
- Common toxicity criteria. Version 2.0. 1999. [cited December 26, 2022]. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf
- Virik K, Glare P. Validation of the palliative performance scale for inpatients admitted to a palliative care unit in Sydney, Australia. J Pain Symptom Manage 2002;23:455-7. [Crossref] [PubMed]
- Masman AD, Tibboel D, Baar FP, et al. Prevalence and Implications of Abnormal Laboratory Results in Patients in the Terminal Phase of Life. J Palliat Med 2016;19:822-9. [Crossref] [PubMed]
- Pirovano M, Maltoni M, Nanni O, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage 1999;17:231-9. [Crossref] [PubMed]
- Gwilliam B, Keeley V, Todd C, et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ 2011;343:d4920. [Crossref] [PubMed]
- Nazha B, Moussaly E, Zaarour M, et al. Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World J Gastrointest Surg 2015;7:370-7. [Crossref] [PubMed]
- Shrotriya S, Walsh D, Bennani-Baiti N, et al. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS One 2015;10:e0143080. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 2015;54:961-70. [Crossref] [PubMed]
- Ohta T, Waga S, Handa W, et al. New grading of level of disordered onsciousness (author’s transl). No Shinkei Geka 1974;2:623-7. [PubMed]
- Maezawa Y, Aoyama T, Kano K, et al. Impact of the Age-adjusted Charlson comorbidity index on the short- and long-term outcomes of patients undergoing curative gastrectomy for gastric cancer. J Cancer 2019;10:5527-35. [Crossref] [PubMed]
- Kahl A, du Bois A, Harter P, et al. Prognostic Value of the Age-Adjusted Charlson Comorbidity Index (ACCI) on Short- and Long-Term Outcome in Patients with Advanced Primary Epithelial Ovarian Cancer. Ann Surg Oncol 2017;24:3692-9. [Crossref] [PubMed]
- Ministry of Health, Labour and Welfare. A report of the 2017 attitude survey on health care at the terminal stage of life. [cited October 10, 2021]. Available online: https://www.mhlw.go.jp/toukei/list/dl/saisyuiryo_a_h29.pdf
- Patel A, Dunmore-Griffith J, Lutz S, et al. Radiation therapy in the last month of life. Rep Pract Oncol Radiother 2013;19:191-4. [Crossref] [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer 2014;120:134-41. [Crossref] [PubMed]
- Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol 2008;26:5863-9. [Crossref] [PubMed]
- Porcu L, Recchia A, Bosetti C, et al. Development and external validation of a predictive multivariable model for last-weeks survival of advanced cancer patients in the palliative home care setting (PACS). Support Care Cancer 2023;31:536. [Crossref] [PubMed]
- Hui D. Unexpected death in palliative care: what to expect when you are not expecting. Curr Opin Support Palliat Care 2015;9:369-74. [Crossref] [PubMed]
- Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol 2011;29:1151-8. [Crossref] [PubMed]
- Rier HN, Jager A, Sleijfer S, et al. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 2016;21:1396-409. [Crossref] [PubMed]
- Nakamura N, Hara T, Shibata Y, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol 2015;94:2043-53. [Crossref] [PubMed]
- Ishida T, Makino T, Yamasaki M, et al. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery 2019;166:1041-7. [Crossref] [PubMed]
- Pak S, Park SY, Shin TJ, et al. Association of Muscle Mass with Survival after Radical Prostatectomy in Patients with Prostate Cancer. J Urol 2019;202:525-32. [Crossref] [PubMed]
- Wang S, Xie H, Gong Y, et al. The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci Rep 2020;10:8153. [Crossref] [PubMed]
- Ferini G, Cacciola A, Parisi S, et al. Curative Radiotherapy in Elderly Patients With Muscle Invasive Bladder Cancer: The Prognostic Role of Sarcopenia. In Vivo 2021;35:571-8. [Crossref] [PubMed]
- McGuire DK, Levine BD, Williamson JW, et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 2001;104:1350-7. [Crossref] [PubMed]
- Wei J, Zeng L, Li S, et al. Relationship between comorbidities and treatment decision-making in elderly hip fracture patients. Aging Clin Exp Res 2019;31:1735-41. [Crossref] [PubMed]
- Bollen L, Jacobs WCH, Van der Linden YM, et al. A systematic review of prognostic factors predicting survival in patients with spinal bone metastases. Eur Spine J 2018;27:799-805. [Crossref] [PubMed]


