
Opioid-induced respiratory depression suspected of drug interaction in a prostate cancer patient: a case report
Highlight box
Key findings
• We encountered a case in which oxycodone had accumulated due to renal dysfunction, and a drug interaction with antiemetics especially aprepitant caused the blood concentration of oxycodone to rise above the acceptable level, resulting in respiratory depression.
What is known and what is new?
• Opioid analgesics metabolized by cytochrome P450 (CYP) require attention regarding their drug interactions with concomitantly administered drugs. Oxycodone is metabolized by CYP3A4, and aprepitant is a CYP3A4 inhibitor.
• If a drug that can inhibit CYP3A4 is administered when the blood concentration of oxycodone is already higher than necessary, there is a possibility that the blood concentration will rise beyond the acceptable level due to a drug interaction.
What is the implication, and what should change now?
• Since terminally ill cancer patients are often given multiple drugs, drug interactions, including opioids, are more likely to occur. Therefore, the clinical significance of drug interactions must be carefully considered in palliative care settings.
Introduction
Opioid analgesics used in clinical practice are metabolized by either cytochrome P450 (CYP) or glucuronide conjugation (1). For example, oxycodone, one of the most commonly used opioid analgesics for cancer pain, is mainly metabolized by CYP3A4, one of the isoenzymes of CYPs (2). In addition to these opioids, many drugs used to treat and alleviate symptoms in cancer patients are metabolized by CYPs, while many drugs also inhibit or induce CYPs (3). Therefore, when using opioids that are metabolized by CYP, it is necessary to pay attention to the possibility that the effects of the opioids may be attenuated or enhanced by their concomitant use with drugs that induce or inhibit CYP (4). Aprepitant, an antiemetic drug used in many patients receiving anticancer drugs, is known to inhibit CYP3A4 (5), and the possibility of its drug interactions with anticancer drugs, opioid analgesics, anticoagulants, and many other drugs that are metabolized by CYP has been pointed out (6). Here, we report a case of respiratory depression in a patient owing to oversedation after the administration of antiemetic drugs prior to anticancer drugs during the administration of oxycodone for cancer pain. We present this case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-581/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
The patient was a 72-year-old Asian man. He was diagnosed as having prostate cancer, multiple bone and lymph node metastases, and rectal invasion. Renal dysfunction was observed from the time of admission and persisted throughout the hospital stay [plasma creatinine: 1.3–1.5 mg/dL, estimated glomerular filtration rate (eGFR): 35–40 mL/min]. No other comorbidities were observed. Treatment for prostate cancer (degarelix and abiraterone) was started. Four months subsequently, he was referred to our palliative care team for pain control. His chief complaint was penetrating pain in the anal region possibly caused by rectal invasion of prostate cancer, and he had already been treated with tramadol (25 mg orally 4 times daily). No other medications have been administered. The oral administration of tramadol reduced his pain from 10/10 to 5–6/10 on the numeric rating scale (NRS), in which 0 represents no pain and 10 represents the worst pain imaginable, but the effect was insufficient. Therefore, we changed the opioid analgesic from tramadol to fentanyl infusion and began titration. When the pain decreased with 0.36 mg/day of fentanyl, we switched to transdermal fentanyl (12.5 µg/h) and 2.5 mg/dose of oxycodone rapid-release formulation as the rescue medication. His pain decreased with 37.5 µg/h of transdermal fentanyl and a 5 mg/dose of oxycodone rapid-release formulation, and the patient was temporarily discharged from the hospital. However, 1 week after discharge, the pain worsened, and he was readmitted to the hospital. After hospitalization, we switched from transdermal fentanyl and oral oxycodone to oxycodone infusion started at 48 mg/day (continuous infusion plus bolus of 1 hour quantity as a rescue dose), and the dose was increased as appropriate while observing the intensity of his pain and the number of times of rescue medication administration, and the pain decreased when the dose was increased to 108 mg/day (Figure 1). On day 42 of rehospitalization, it was determined that the pain was well controlled, and anticancer drugs were scheduled to be administered.
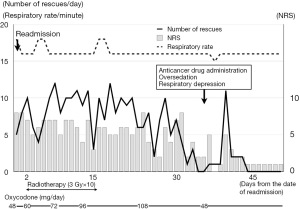
Three antiemetic drugs [dexamethasone (9.9 mg), palonosetron (0.75 mg), and fosaprepitant (150 mg)] were successively administered intravenously prior to anticancer drug administration (fosaprepitant is rapidly metabolized to aprepitant after intravenous administration). About 3 hours after the administration, the ward nurse noticed that the patient was more sleepy than usual, and when he fell asleep, his breathing rate decreased to about 6 times per minute (Figure 2). When he was called, he immediately woke up and was able to have a normal conversation. Therefore, anticancer drugs [cisplatin (90 mg) and etoposide (140 mg)] were administered as planned. About 2 hours after the nurse noticed this oversedation, the attending physician examined the patient and reduced the dose of oxycodone infusion to 48 mg/day. Subsequently, although his drowsiness persisted, his respiratory condition improved. The next morning, he was able to talk as usual, was drowsy, but his breathing was stable, and despite reducing the dose of oxycodone to less than half, he had an NRS score of 0–1, and his pain had subsided without the use of any rescue medication. On the second and third days, only dexamethasone was administered as an antiemetic, followed by etoposide, but no problems occurred. Thereafter, his NRS score continued to be 0–1/10, and 27 days after the administration of anticancer drugs, oxycodone infusion was switched to an oral drug [oxycodone sustained-release preparation (60 mg/day)]. Even after this change, the patient continued to maintain an NRS score of 0 without using any rescue medication, and he was discharged from the hospital 36 days after the administration of anticancer drugs, without any problems.

Discussion
In the present case, the patient’s pain was well controlled with oxycodone until the morning of the administration of the anticancer drugs, without any major side effects. However, after the administration of the antiemetics prior to the anticancer drugs, sudden deterioration of his consciousness level and respiratory depression occurred. His respiratory rate recovered upon a dose reduction of oxycodone. The cause of the decreased level of consciousness and respiratory depression is unclear, but the timing and course of events most likely suggest a drug interaction between oxycodone and the antiemetics, given that no other medications were administered that could affect level of consciousness or breathing. Drug interaction probability scale demonstrated by Horn et al. (7) indicates the score “possible” in this case. Oxycodone is predominantly metabolized by N-demethylation via CYP3A4/5 to noroxycodone, which is a relatively weak active metabolite with low potency for the µ opioid receptor; oxycodone is also metabolized by O-demethylation via CYP2D6 to oxymorphone, which is its main active metabolite predominantly responsible for the analgesic effect of oxycodone (4). On the other hand, aprepitant is a moderate substrate of CYP3A4, whereby it competitively inhibits the metabolism of an opioid analgesic that is a weaker substrate of CYP3A4 such as oxycodone. Among other antiemetics used in this case, dexamethasone is categorized to be a strong CYP inducer (8), and the risk of central nervous system (CNS) depression can be increased when palonosetron is combined with oxycodone as serotonin syndrome (Drug Interaction Checker: https://www.drugs.com/drug_interactions.html). Therefore, although all of these are at risk for drug interactions with oxycodone, aprepitant appears to be the one most likely associated with the cause of oversedation and respiratory depression in this case. On the other hand, the number of rescue medication doses had decreased from several days before the event (Figure 1), suggesting that the effects of radiation therapy (3 Gy dose 10 times for 2 weeks, starting 4 weeks before the administration of anti-cancer drugs) (Figure 1) had already reduced the pain. In other words, it is possible that it was already time to reduce the dose of oxycodone, but we continued the same dose resulting in an overdose. Antiemetics especially aprepitant may have inhibited the metabolism of oxycodone in this condition. As a result, blood levels may have risen above acceptable levels. In fact, even after reducing the dose of oxycodone, the pain was well controlled with little need for rescue medication. Furthermore, because this patient’s renal function had declined throughout his hospitalization, oxycodone and its metabolites may have accumulated, which may also have contributed in some degree to the resulting oversedation and respiratory depression.
Fujiwara et al. (9) measured the course of oxycodone blood concentrations when aprepitant was coadministered to patients taking oxycodone and showed that oxycodone blood concentrations significantly increased upon the coadministration of aprepitant. However, they concluded that when used with appropriate caution, there appeared to be no need to change the dose of oxycodone in clinical practice. Oxycodone was administered orally in their study, whereas it was administered parenterally in the present case. Parenteral administration avoids gastrointestinal (GI) tract, where CYP3A4/5 enzymes are known to be in abundance, and bypasses first-pass hepatic metabolism. Moreover, the dosage is also quite different from the present case. Therefore, the data shown by Fujiwara et al. cannot be directly applied to the present case. They also showed that oxymorphone, active metabolite of oxycodone, also increased significantly by 34%. It is thus possible that increased blood levels of both oxycodone and oxymorphone are contributing to this respiratory depression, but this remains speculation as blood levels were not measured. Since terminally ill cancer patients are often given multiple drugs, drug interactions, including opioids, are more likely to occur (10). Therefore, the clinical importance of drug interactions must be considered with great care in palliative care settings.
Conclusions
We encountered a case of a patient in whom oversedation and respiratory depression occurred when antiemetics were administered before anticancer drugs administration during pain control with oxycodone. The cause is assumed to be a combination of factors, including the possibility that the metabolism of oxycodone was inhibited due to drug interactions caused by the administration of multiple antiemetics especially aprepitant, the possibility that it was originally time to reduce the dose of oxycodone, and the possibility that oxycodone accumulated due to renal dysfunction.
Acknowledgments
We would like to thank Center for International Education and Research at Tokyo Medical University for English proofreading.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-581/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-581/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-581/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trescot AM, Datta S, Lee M, et al. Opioid pharmacology. Pain Physician 2008;11:S133-53. [Crossref] [PubMed]
- Lalovic B, Phillips B, Risler LL, et al. Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos 2004;32:447-54. [Crossref] [PubMed]
- Fujita K. Cytochrome P450 and anticancer drugs. Curr Drug Metab 2006;7:23-37. [Crossref] [PubMed]
- Coates S, Lazarus P. Hydrocodone, Oxycodone, and Morphine Metabolism and Drug-Drug Interactions. J Pharmacol Exp Ther 2023;387:150-69. [Crossref] [PubMed]
- Sanchez RI, Wang RW, Newton DJ, et al. Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos 2004;32:1287-92. [Crossref] [PubMed]
- Dushenkov A, Kalabalik J, Carbone A, et al. Drug interactions with aprepitant or fosaprepitant: Review of literature and implications for clinical practice. J Oncol Pharm Pract 2017;23:296-308. [Crossref] [PubMed]
- Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother 2007;41:674-80. [Crossref] [PubMed]
- Bourdin V, Bigot W, Vanjak A, et al. Drug-Drug Interactions Involving Dexamethasone in Clinical Practice: Myth or Reality? J Clin Med 2023;12:7120. [Crossref] [PubMed]
- Fujiwara Y, Toyoda M, Chayahara N, et al. Effects of aprepitant on the pharmacokinetics of controlled-release oral oxycodone in cancer patients. PLoS One 2014;9:e104215. [Crossref] [PubMed]
- Mahzoni H, Naghsh E, Sharifi M, et al. Potential Drug Interactions in Terminally-Ill Cancer Patients, a Report from the Middle East. J Pain Palliat Care Pharmacother 2023;37:278-85. [Crossref] [PubMed]


