
Treatment of localized hepatocellular carcinoma: resection vs. ablation vs. radiation
Introduction to hepatocellular carcinoma (HCC)
HCC is the sixth most commonly diagnosed cancer worldwide (1). It is the third leading cause of cancer death worldwide and the second leading cause of cancer death among men (1). HCC is particularly common in eastern Asia (2,3). Risk factors for HCC include hepatitis B virus (HBV), hepatitis C virus (HCV), aflatoxin-contaminated foods, heavy alcohol intake, excess body weight, type 2 diabetes mellitus (T2DM), and smoking (4).
According to Surveillance, Epidemiology, and End Results (SEER) data for liver and intrahepatic bile duct cancer in 2019, 39% of new HCC diagnoses are localized at the time of diagnosis and with the implementation of more screening surveillance there may be an increase in the number of localized HCC diagnosis (5,6). Finding the best way to treat these early HCCs is essential since recurrence of disease usually results in cancer-specific mortality. The mean overall survival (OS) of untreated HCC is generally reported to be 4 to 6 months after symptoms appear (7). To avoid this rapid progression, potentially curative treatment of localized HCC must be initiated rapidly and providers must be up to date on the most effective and safe treatment modalities. The ability of a treatment to avoid damaging healthy liver is a major consideration given that over 90% of HCC cases occur in the setting of chronic liver disease and compromised liver function (8).
Barcelona Clinic Liver Cancer (BCLC) staging
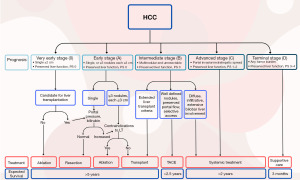
Accurate staging is essential for a discussion of treatment options for patients with HCC. Many staging systems have been developed. The most widely adopted method is the BCLC staging system, which considers the characteristics of the tumor, underlying liver disease, and patient’s performance status. The BCLC system guidelines link the stage with recommended treatment strategies and are also adopted by the National Comprehensive Cancer Network (NCCN) in their guidelines. The updated guidelines as of 2022 (9) are depicted in Figure 1. The figure demonstrates that the primary management for early-stage disease involves ablation for very early stage (BCLC-0) or less than 2 cm. With larger lesions (under 5 cm) or up to three lesions, hepatectomy or transplantation are reasonable options.
Ablative techniques
Ablation refers to treatment that destroys tumor tissue without removing it. Broadly the term includes treatment with ablative doses of external beam radiation but usually it refers specifically to noninvasive or minimally invasive/percutaneous techniques including ethanol ablation, radiofrequency ablation (RFA), microwave ablation (MWA), laser ablation, cryoablation.
Ethanol ablation
One of the earliest forms of percutaneous tumor ablation is ethanol injection. First described in 1983, percutaneous ethanol injection (PEI) uses ultrasound or computed tomography (CT) imaging to guide a needle into a tumor (10). Concentrated ethanol is injected and causes direct damage to tumor cells via dehydration and denaturation leading to coagulative necrosis. It is a safe, easy to perform, cost-effective treatment. Only small lesions can be effectively treated with this technique and it often requires multiple sessions to ensure that ethanol diffusion reaches each part of the tumor.
Thermal ablation
The thermal ablative therapies are either hyperthermic treatments—including RFA, MWA, and laser ablation—or cryoablation. Hyperthermic treatments produce irreversible cellular damage, coagulation of tissue, and damage to mitochondrial and cytosolic enzymes in cells. Cryoablation kills primarily via cell membrane disruption after cooling to temperatures between −20 and −60 ℃ followed by rapid thawing.
RFA is a minimally invasive hyperthermic ablative technique using heat generated from an electrical current. After general anesthesia is induced, a thin needle electrode is inserted into the tumor through the skin and ground pads are placed on a separate portion of skin (11). An electric current that oscillates at radio frequencies is conducted from the needle to the ground pads with the patient creating a closed loop circuit. The high electrical resistance in the tissue surrounding the thin needle electrode causes heat buildup and eventual thermal injury in close proximity to the needle. The larger surface area of the ground pads placed on the skin dissipates the energy to avoid skin damage. Generally, RFA is indicated for BCLC stage 0 and in properly selected BCLC stage A.
MWA refers to all techniques of tumor destruction that use devices that generate electromagnetic radiation (EMR) with frequencies ≥900 kHz. This lower energy radiation does not induce DNA damage like ionizing radiation used in stereotactic body radiation therapy (SBRT) but instead generates heat as it passes through tissue. This causes thermal injury that drops off rapidly as distance from the source increases. This falls into the same category of thermal ablation as RFA. Importantly, vessels located in the proximity of the tumor are not as much at risk with MWA as they are with RFA.
The third method of hyperthermia ablation is laser ablation, which uses light energy applied via optical fibers inserted into tissue. This energy is absorbed into tissue creating temperatures of up to 150 ℃. There is less data regarding laser ablation than regarding RFA and MWA.
Cryoablation is a technique wherein a probe cooled with liquid nitrogen is placed into the tumor and an ice ball is created in the target tissue. The technique has limited application in HCC (12,13). The complication rate is not negligible, particularly because of the risk for “cryoshock”, a life-threatening condition resulting in multiorgan failure, severe coagulopathy, and disseminated intravascular coagulation following cryoablation. There are currently no randomized controlled trials (RCTs) that support the use of hepatic cryoablation for HCC treatment.
Comparison of ablative techniques
According to BCLC staging, for single lesions of less than or equal to 2 cm greatest dimension with preserved liver function, and good performance status, resection and ablation are both standard options. However, as we will see below, there is some data to argue that resection upfront may decrease local recurrence risk even in these stage 0 patients. Therefore, ablation is not widely considered standard of care at this time for upfront treatment for patients with good functional status and liver function unless there are two or three small lesions that will make surgery unfeasible.
RF vs. ethanol
PEI was the first ablative technique widely adopted but in modern years, it has fallen out of favor due to many clinical trials that have demonstrated the superiority of RFA, which is now the most widely adopted technique. Several clinical trials have shown increased antitumor effect and locoregional disease control with RFA over PEI (11,14-17).
Brunello et al.’s 2008 publication (17) is a good representation of the results of these five trials. They found finding in an intention-to-treat analysis that complete response (CR) at 1 year was achieved by 65.7% and 36.2% of patients treated by RFA and PEI, respectively in early HCC (P=0.0005).
Complications occurred in 10 and 12 patients treated by RFA and PEI, respectively (17). In patients with cirrhosis, RFA did not provide a clear survival advantage.
The impact of RFA on OS is not as well established as its impact on locoregional control but the three meta-analyses below demonstrate an OS benefit particularly for tumors larger than 2 cm (18-20).
Orlando et al. in 2009 analyzed five trials with 701 total patients with small HCC and found that OS was significantly higher in patients treated with RF than in those treated with PEI with odds ratio of 1.92 (1.35–2.74) (18). This data agrees with Cho et al. who published a meta-analysis in the same year of 4 of the same clinical trials showing significant improvement in 3-year OS favoring RFA [odds ratio, 0.477 (0.340–0.670)] (19). The next year, Germani et al. published a third meta-analysis including trials with percutaneous acetic acid injection (PAI) as well as PEI analyzing 1,035 patients showing that RFA was superior to PEI for survival with OR 0.52 (0.35–0.78) but only for tumors greater than 2 cm. PAI did not significantly differ from PEI for survival and local recurrence but required less sessions (20).
Overall, this data confirms that RFA is superior to PEI.
MWA vs. RFA
Randomized data is limited in comparing MWA with RFA. One of the largest trials published by Kamal et al. showed no statistically significant differences were observed with respect to the efficacy of the two procedures, but there was a slight trend favoring RFA with respect to complication rates (21). A meta-analysis published in 2020 by Facciorusso et al. shows a similar efficacy and safety profile between the two techniques (22). There was a trend toward a decrease in long-term recurrences with MWA but this needs to be studied further.
A phase II clinical trial shows that MWA and RFA show similar diameters of ablative regions, similar OS, and similar recurrence-free survival (RFS) at 50 months in liver tumors between 1.5 and 4 cm (23). Hospital and treatment costs become more important when two treatments appear to be equally effective. Some studies have found that MWA is cheaper than RFA and requires fewer treatment sessions (24) and shorter overall treatment durations (25,26). Of note, MWA technology has evolved significantly since the publication of this trial. Newer devices can achieve higher temperatures and a larger volume of coagulation (27). Further studies are needed to compare MWA to RFA and resection in patients with larger volume of disease. Considering the data that we currently have, both MWA and RFA are very suitable options for percutaneous ablation of liver tumors.
RFA vs. laser ablation
Many studies demonstrate local recurrence and median survival rates with laser ablation that are comparable to those with RFA (28-30). There are two randomized clinical trials comparing laser ablation and RFA in the treatment of HCC with no significant difference between the two arms in terms of tumor control, OS, and safety although Ferrari et al. found on univariate analysis that those with Child-Pugh A or tumors <2.5 cm or single lesions seem to benefit more from RFA than laser ablation with significant improvement in OS for each of these variables favoring RFA (31,32). Laser ablation is an acceptable alternative to RFA but is not usually considered a standard option. No RCTs to compare laser ablation with non-RFA forms of ablation have been published.
RFA has been shown to be a very useful tool in treating early HCC and is cost-effective and likely represents the best that our current technology can achieve percutaneously. Outcomes with RFA cannot be beaten by PEI, MWA, or laser ablation as far as the data show currently and so it continues to be the gold standard for local percutaneous treatment.
Surgical resection (SR)
SR or transplantation for stage 0 and stage A HCC is the current standard of care for patients who are fit for surgery. Unfortunately, HCC is commonly set in the background of poor liver function and many patients are not amenable to SR upfront (8). In patients with stage I/II disease, 5-year survival rates of 60–70% have been reported compared to 20–30% in patients with stage III/IV disease (33,34). Additionally, operative mortality rate from liver failure after hepatectomy ranges from 0 to 32% (35-39) but the rate may decrease with hospital experience and as our treatments become more optimized (40). For small tumors, less than 3 cm, RFA can be used for definitive management.
Resection vs. ablation
For very early-stage HCC, there is a wealth of data with large numbers of patients that supports the use of resection over RFA.
Chu et al. retrospectively reviewed 1,208 patients and found 15-year OS rates of 60.4% and 51.6% in the resection and RFA groups respectively (P=0.0378). The 15-year RFS rates were 37% and 23.6% in the resection and RFA groups, respectively (P<0.001) (41). Inherently, retrospective data can be problematic when one of the treatment options (resection) is only considered for patients with good liver function and performance status, introducing bias that can explain their prolonged survival. However, survival benefits can still be found in prospective data. A 2020 meta-analysis of RFA vs. SR for all resectable HCC analyzed 13,147 patients. In total, 6,727 were treated with RFA and 6,420 were treated with SR. The OS rates and disease-free survival (DFS) rates were improved with SR over RFA with odds ratios of 0.566 and 0.374 respectively at 5 years. For tumors >3 cm, the OS and DFS were not improved with SR vs. RFA (42). At least for small tumors, it seems that SR is superior to RFA for HCC in a patient who is a good surgical candidate.
Table 1 shows multiple trials in resection vs. RFA that have been able to prospectively compare patients with similar tumor sizes and Child-Pugh scores (43-48).
Table 1
| Studies | Country | Patient number | CTP score | Tumor size | Tumor number | OS | RFS |
|---|---|---|---|---|---|---|---|
| Chen (43) | China | 71 vs. 90 | A vs. A | ≤5 cm | 1 | NS | NS |
| Huang (44) | China | 115 vs. 115 | A, B vs. A, B | ≤5 cm | ≤3 | Better in LR | Better in LR |
| Feng (45) | China | 84 vs. 84 | A, B vs. A, B | ≤4 cm | ≤2 | NS | NS |
| Fang (46) | China | 60 vs. 60 | A, B vs. A, B, C (5) | ≤3 cm | ≤3 | NS | NS |
| Ng (47) | Japan | 109 vs. 109 | A, B vs. A, B | ≤5 cm | ≤3 | NS | NS |
| Lee (48) | Korea | 29 vs. 34 | A vs. A | 2–4 cm | 1 | NS | Better in LR |
Most of these trials demonstrate a trend toward better RFS with resection over RFA. Huang et al. reports an improvement in OS as well (44). RFA, radiofrequency ablation; CTP, Child-Turcotte-Pugh; OS, overall survival; RFS, recurrence-free survival; NS, not significant; LR, liver resection.
Of note, RFA is generally more cost-effective than resection as demonstrated by Cucchetti et al. in 2013 (49).
Resection vs. MWA
Limited data exists to compare surgery to MWA. Nonrandomized data from 2019 Liu et al. reviewing outcomes in resection vs. MWA shows a trend toward better outcomes with resection. They showed statistically significant improvement in RFS with resection over RFA. The trial included 212 patients in the resection arm vs. 116 in the MWA arm with tumor size less than or equal to 5 cm and less than or equal to three tumors. RFS was 30.6% vs. 57.5% in the liver resection and MWA arms respectively (P<0.001). OS at 5 years was similar in both arms (82.2% vs. 80.5% in the resection and MWA arms respectively (P=0.360) (50). Modern MWA techniques are rapidly evolving and prospective data to compare these techniques to the standard of care is needed.
There is not strong data comparing resection to other forms of ablation but due to its superiority over RFA, we can extrapolate that no other form of ablation currently available will be preferred when the patient can tolerate surgery. Overall, surgery gives the patient a better chance at local control but with advancements in ablation technology and with the large subset of patients who present with unresectable status, the debate regarding resection vs. ablation will stay relevant for early-stage HCC for some time.
Stereotactic ablative radiation
External beam radiation can be offered for HCC patients and while techniques have evolved over the years, it has been an important tool for treating HCC for many years. Historical treatment techniques often led to large volumes of liver being encompassed in the radiation field. Thus, a limiting factor for EBRT use was the development of radiation-induced liver disease (RILD) which occurred in around 10% of cases and has been shown to be dependent on total volume irradiated and dose (51,52). However, with advancements in technology, such as intensity-modulated radiation therapy (IMRT), and image-guided radiation therapy (IGRT) modern radiation oncologists have the ability to administer high doses of radiation to the liver without any significant risk of RILD (53). SBRT is a highly conformal external beam radiation technique that delivers ablative doses in a few fractions. High biologically effective dose to tumor results in high likelihood of cell kill and tumor control and with high conformality and sharp dose drop off the surrounding liver can usually be safely spared.
Residual liver tissue is the primary organ at risk with SBRT and while SBRT is the least invasive technique currently available, the risk of damaging surrounding liver is higher than other local ablative therapies. Preservation of liver function is usually achievable depending on total liver volume, volume of lesions, prior treatments, and baseline liver function. Patients with liver cirrhosis Child-Pugh class A and early B are usually considered suitable candidates. Lesions close to large vessels or with portal vein thrombosis are usually better candidates for SBRT than for RFA. On the other hand, lesions adjacent to organs that are more radiosensitive, such as small bowel and stomach, may not be good SBRT candidates as dose reduction to achieve dose constraints may compromise tumor control. Even in these scenarios, though HCC is a radiosensitive disease and dose reductions can still yield considerable local control benefits.
Adequate pretreatment imaging to aid in treatment planning, patient immobilization, and motion management, as well as image guidance for daily treatments are all necessary to perform accurate SBRT. Often, fiducial markers are placed to aid with identifying the lesion on planning imaging and for daily treatments to ensure accurate targeting.
In recent years, SBRT has shown excellent results in local control of HCC with low toxicity and is being used more and more frequently but its role is still being elucidated. In the past SBRT had been preserved primarily for use in patients with 1–3 liver lesions with a maximum diameter ≥5 cm who are not eligible for resection. More recently it has been explored as a bridging strategy for patients awaiting liver transplants as well as in combination with other ablative therapies. It is essential to evaluate SBRT head-to-head with other local therapy modalities in early-stage HCC.
One of the first publications to provide rationale for further investigation of SBRT in HCC with randomized trials came from Bujold et al. in 2013 (53). In a phase II trial, 102 patients were analyzed with Child-Turcotte-Pugh class A disease, with at least 700 mL of non-HCC liver. The SBRT dose range was 24 to 54 Gy in 6 fractions, which was adjusted based on proximity to luminal gastrointestinal (GI) organs or total liver volume. Primary endpoints were toxicity and local control at 1 year (LC1y). A total of 102 patients were evaluated. Tumor-node-metastasis (TNM) stage was III in 66%, and 61% had multiple lesions. Median gross tumor volume was 117.0 mL (range, 1.3 to 1,913.4 mL). Tumor vascular thrombosis (TVT) was present in 55%, and extrahepatic disease was present in 12%. Despite these advanced features, local control at 1-year was 87%. There was a relationship between SBRT dose [hazard ratio (HR), 0.96; P=0.02] and local control. Patients who received 30 Gy over had a local control of 85% vs. 66% at 2-year follow-up. Toxicity ≥ grade 3 was seen in 30% of patients. In seven patients (two with TVT PD), death was possibly related to treatment (1.1 to 7.7 months after SBRT). Median OS was 17.0 months [95% confidence interval (CI): 10.4 to 21.3 months]. These data showed some promise that good local control and survival could be obtained from SBRT even in patients with more advanced and bulky burden of disease.
SBRT vs. RFA
Wahl et al. [2016] published a retrospective review of outcomes in patients receiving SBRT vs. RFA. 224 patients with inoperable, nonmetastatic HCC underwent RFA (n=161) to 249 tumors or image-guided SBRT (n=63) to 83 tumors. Freedom from local progression (FFLP) and toxicity were retrospectively analyzed. However, the two groups were not equivalent at baseline as the SBRT group had lower pretreatment Child-Pugh scores (P=0.003), higher pretreatment alpha-fetoprotein levels (P=0.04), and a greater number of prior liver-directed treatments (P<0.001). One- and 2-year FFLP for tumors treated with RFA were 83.6% and 80.2% vs. 97.4% and 83.8% for SBRT. For tumors ≥2 cm, FFLP was worse for RFA compared with SBRT (HR, 3.35; P=0.025). Acute grade 3+ complications did not significantly differ. OS was not significantly different at 1 and 2 years after treatment with values of 70% and 53% after RFA and 74% and 46% after SBRT (54). Unlike RFA, SBRT is not susceptible to the heat sink effect of being near vessels and 100% of the tumor can objectively and volumetrically be prescribed too (55).
For many patients with stage A HCC, transplantation is ideal and those on the waiting list for liver transplantation are at risk of tumor progression and death. Bridging therapy is therefore essential and is aimed at controlling disease until the time of transplantation. RFA and transarterial chemoembolization (TACE) are established bridging therapies. A retrospective series, Sapisochin et al. provided good evidence for SBRT as a bridging therapy as compared to RFA and TACE (56). The authors aimed to ascertain the safety and efficacy of SBRT on an intention-to-treat basis compared with TACE and RFA as a bridge to liver transplantation in a large cohort of patients with HCC. A total of 379 patients were treated with either SBRT (n=36), TACE (n=99), or RFA (n=244). Thirty patients were transplanted in the SBRT group, 79 in the TACE group, and 203 in the RFA group. Postoperative complications were similar between groups. Patients in the RFA group had more tumor necrosis in the explant. The 1-, 3-, and 5-year actuarial patient survival from the time of listing was 83%, 61%, and 61% in the SBRT group vs. 86%, 61%, and 56% in the TACE group, and 86%, 72%, and 61% in the RFA group, P=0.4. The 1-, 3-, and 5-year survival from the time of transplant was 83%, 75%, and 75% in the SBRT group vs. 96%, 75%, and 69% in the TACE group, and 95%, 81%, and 73% in the RFA group, P=0.7. The authors concluded that SBRT can be safely utilized as a bridge to LT in patients with HCC, as an alternative to conventional bridging therapies.
These retrospective data suggest that both RFA and SBRT are effective local treatment options for inoperable HCC. It is important to note though that with larger sizes, SBRT is not merely a “dichotomous” effect for liver cancer. While RFA would be considered a dichotomous modality of either being administered or not, SBRT has the advantage of knowing objectively the dose on a continuous scale. No matter the size, one can have confidence that an ablative dose was administered. This data may pave the way for SBRT to be studied prospectively and potentially gain a place as a reasonable first-line treatment option in early HCC.
SBRT vs. TACE
There have been several studies investigating the role of SBRT vs. TACE. Consistently across multiple randomized trials, SBRT has demonstrated to have a superior local control effect although it does not have a survival benefit. It also has less adverse effects, and it is potentially less costly than TACE. There was a large retrospective study from Michigan which demonstrated this. It was shown that in Sapir retrospectively, in a large review, etc. that when comparing TACE vs. SBRT, the 1- and 2-year local control were 97% and 91% for SBRT while they were 47% and 23% for TACE (HR, 66.5; P<0.001) (57). Of note, if there was segmental portal vein invasion SBRT controlled the disease. This series has been confirmed by multiple separate randomized clinical trials. The trendy trial was a randomized phase 2 study comparing TACE to SBRT. In this study, the median local control in the TACE arm was 12 months while in the SBRT it was not reached at 40 months. Additionally, the median time to progression was 12 months for TACE vs. 19 months for SBRT (58). Grade 3 adverse events were 0% in SBRT vs. 13% in TACE. There was no difference in survival. Another randomized evaluated the difference between proton therapy and TACE in patients suitable for transplantation. In this trial, it was found that local control was improved with protons was improved vs. TACE (HR, 5.64; P=0.003). The progression-free survival (PFS) median was 12 months for TACE vs. not reached for proton therapy. This trial also found a reduction in hospitalizations which were 24 for proton beam radiotherapy (PBT) vs. 166 for TACE (P<0.001). Proton beam therapy, which is generally more costly than the more widely used photon-based SBRT, was 28% less costly than TACE (59). Another randomized trial demonstrated that after a failure of TACE, there was a marked benefit to utilizing SBRT over TACE. The median local control was not reached in the SBRT vs. being a median of 8 months in the TACE arm. One-year local control was 84% in SBRT arm vs. 23% in the TACE arm. There was no difference in OS as well. The patients treated with SBRT was significantly longer PFS in comparison with patients treated with TACE with a median of 9 vs. 4 months (60).
Locally advanced HCC
Utilizing SBRT for locally advanced disease is a current option with level 1 evidence. For tumors that are ineligible for transplantation and locally advanced, SBRT has a large survival benefit. The use of SBRT is naturally attractive as a noninvasive yet effective option for patients with higher burden of disease where resection and even ablation would not be feasible. This is demonstrated by the recently published abstract for RTOG 1112 in 2022 showing a survival benefit to adding SBRT to sorafenib in new or recurrent locally advanced HCC that are ineligible for other local treatment modalities (61).
The authors hypothesized that OS would improve with SBRT followed by sorafenib vs. sorafenib alone in patients with advanced HCC. Patients had Child-Pugh A with maximum HCC volume 20 cm and no more than three extrahepatic metastases. Patients were randomized 1:1 to sorafenib vs. SBRT (27.5–50 Gy in 5 fractions, with dose individualized based on liver and other constraints) followed by sorafenib. A total of 177 patients were randomized to the two arms. Most patients (82%) had BCLC stage C disease with macrovascular invasion in 74%. Amongst the vascular invasions the vast majority had either VP3 (main portal branch) or VP4 (main portal vein invasion) establishing that this the most advanced patient population evaluated within an HCC clinical trial evaluating a local modality. Median OS was 12.3 months in the sorafenib arm vs. 15.8 months in the SBRT/sorafenib arm. OS was also statistically significantly improved for SBRT/sorafenib after adjusting for performance status, M stage, Child-Pugh A5 vs. 6, and degree of vascular HCC. Median PFS was 5.5 months with sorafenib vs. 9.2 months with SBRT/sorafenib. Grade 3+ AEs were not significantly different. This new data clearly shows the added local control benefit from adding SBRT in these patients with advanced disease to systemic therapy with no additional toxicity than drug therapy alone. This trial also raises the question of whether the immunostimulatory traits of radiation may play a part in the effectiveness of SBRT and may provide a benefit that resection and ablation do not, especially when used in conjunction with immunomodulatory agents.
Immunotherapy
Immunotherapy is an evolving treatment option for many disease sites. While there is a Food and Drug Administration (FDA) indication for atezolimumab and bevacizumab in locally advanced HCC (62), the data to support treating early HCC with immunotherapy is sparse. It is estimated that of all HCC patients to be treated with immune checkpoint inhibitors, about 70% have no response to treatment, perhaps because of the very heterogenous immune microenvironment of HCCs (63,64). Identifying molecular markers that may predict which patients will respond to treatment is an area of ongoing research. Finding the correct combinations of immune modulation and other ablative techniques is of particular interest. One popular theory claims synergism between ablation and immunotherapy dependent on the fact that antigen presentation is increased after an ablative event to the tumor (64). The introduction of immunomodulatory agents timed appropriately with increased antigen spill could lead to a greater immune response. Larger randomized studies are needed to address these questions in HCC.
Conclusions
For early HCC, the treatment paradigm favors resection or transplantation for all who can safely undergo the procedure. For small lesions that can be easily accessed for percutaneous ablation, RFA or MWA are well-studied and effective treatments. For patients where ablation and surgery are not feasible, SBRT appears to offer equivalent if not superior rates of local control depending on the clinical scenario. For those meeting transplant criteria, radiation may offer better local control, PFS, less adverse events, and be less costly than TACE. For locally advanced disease SBRT with the addition of systemic therapy should be the standard of care as this is the only regimen with level 1 evidence for a marked OS benefit.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by Guest Editors (Sukeshi Patel Arora and Sherri Rauenzahn Cervantez) for the series “Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium” published in Annals of Palliative Medicine. The article has undergone external peer review.
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-486/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-486/coif). The series “Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer 2020;147:317-30. [Crossref] [PubMed]
- Valery PC, Laversanne M, Clark PJ, et al. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018;67:600-11. [Crossref] [PubMed]
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021;73:4-13. [Crossref] [PubMed]
- Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer. [Cited 2023 Jun 26]. Available online: https://seer.cancer.gov/statfacts/html/livibd.html
- Petrick JL, Florio AA, Loomba R, et al. Have incidence rates of liver cancer peaked in the United States? Cancer 2020;126:3151-5. [Crossref] [PubMed]
- Parks RW, Garden OJ. Liver resection for cancer. World J Gastroenterol 2001;7:766-71. [Crossref] [PubMed]
- Akriviadis EA, Llovet JM, Efremidis SC, et al. Hepatocellular carcinoma. Br J Surg 1998;85:1319-31. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Sugiura N, Takara K, Ohto M, et al. Percutaneous intratumoral injection of ethanol under ultrasound imaging for treatment of small hepatocellular carcinoma. Acta Hepatol Jpn 1983;24:920-3. [Crossref]
- Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-30. [Crossref] [PubMed]
- Orlacchio A, Bazzocchi G, Pastorelli D, et al. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: initial experience. Cardiovasc Intervent Radiol 2008;31:587-94. [Crossref] [PubMed]
- Shimizu T, Sakuhara Y, Abo D, et al. Outcome of MR-guided percutaneous cryoablation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:816-23. [Crossref] [PubMed]
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-40. [Crossref] [PubMed]
- Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004;127:1714-23. [Crossref] [PubMed]
- Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-6. [Crossref] [PubMed]
- Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol 2008;43:727-35. [Crossref] [PubMed]
- Orlando A, Leandro G, Olivo M, et al. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol 2009;104:514-24. [Crossref] [PubMed]
- Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009;49:453-9. [Crossref] [PubMed]
- Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380-8. [Crossref] [PubMed]
- Kamal A, Elmoety AAA, Rostom YAM, et al. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol 2019;10:562-71. [Crossref] [PubMed]
- Facciorusso A, Abd El Aziz MA, Tartaglia N, et al. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers (Basel) 2020;12:3796. [Crossref] [PubMed]
- Radosevic A, Quesada R, Serlavos C, et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: a randomized controlled phase 2 trial. Sci Rep 2022;12:316. [Crossref] [PubMed]
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009;24:223-7. [Crossref] [PubMed]
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331-7. [Crossref] [PubMed]
- Iida H, Aihara T, Ikuta S, et al. A comparative study of therapeutic effect between laparoscopic microwave coagulation and laparoscopic radiofrequency ablation. Hepatogastroenterology 2013;60:662-5. [PubMed]
- Tamai H, Okamura J. New next-generation microwave thermosphere ablation for small hepatocellular carcinoma. Clin Mol Hepatol 2021;27:564-74. [Crossref] [PubMed]
- Pacella CM, Bizzarri G, Magnolfi F, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology 2001;221:712-20. [Crossref] [PubMed]
- Pacella CM, Francica G, Di Lascio FM, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol 2009;27:2615-21. [Crossref] [PubMed]
- Eichler K, Zangos S, Gruber-Rouh T, et al. Magnetic resonance-guided laser-induced thermotherapy in patients with oligonodular hepatocellular carcinoma: long-term results over a 15-year period. J Clin Gastroenterol 2012;46:796-801. [Crossref] [PubMed]
- Ferrari FS, Megliola A, Scorzelli A, et al. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med 2007;112:377-93. [Crossref] [PubMed]
- Di Costanzo GG, Tortora R, D'Adamo G, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol 2015;30:559-65. [Crossref] [PubMed]
- Ringe B, Pichlmayr R, Wittekind C, et al. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg 1991;15:270-85. [Crossref] [PubMed]
- Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg 1991;214:221-8; discussion 228-9. [Crossref] [PubMed]
- Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-30. [Crossref] [PubMed]
- Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg 1999;134:984-92. [Crossref] [PubMed]
- Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999;229:790-9; discussion 799-800. [Crossref] [PubMed]
- Shuto T, Hirohashi K, Kubo S, et al. Efficacy of major hepatic resection for large hepatocellular carcinoma. Hepatogastroenterology 1999;46:413-6. [PubMed]
- Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-5. [Crossref] [PubMed]
- Glasgow RE, Showstack JA, Katz PP, et al. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg 1999;134:30-5. [Crossref] [PubMed]
- Chu HH, Kim JH, Kim PN, et al. Surgical resection versus radiofrequency ablation very early-stage HCC (≤2 cm Single HCC): A propensity score analysis. Liver Int 2019;39:2397-407. [Crossref] [PubMed]
- Li JK, Liu XH, Cui H, et al. Radiofrequency ablation vs. surgical resection for resectable hepatocellular carcinoma: A systematic review and meta-analysis. Mol Clin Oncol 2020;12:15-22. [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. [Crossref] [PubMed]
- Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:193-200. [Crossref] [PubMed]
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775-84. [Crossref] [PubMed]
- Lee HW, Lee JM, Yoon JH, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res 2018;94:74-82. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300-7. [Crossref] [PubMed]
- Liu W, Zou R, Wang C, et al. Microwave ablation versus resection for hepatocellular carcinoma within the Milan criteria: a propensity-score analysis. Ther Adv Med Oncol 2019;11:1758835919874652. [Crossref] [PubMed]
- Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys 1992;23:781-8. [Crossref] [PubMed]
- Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810-21. [Crossref] [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. [Crossref] [PubMed]
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 2016;34:452-9. [Crossref] [PubMed]
- Lehmann KS, Poch FG, Rieder C, et al. Minimal vascular flows cause strong heat sink effects in hepatic radiofrequency ablation ex vivo. J Hepatobiliary Pancreat Sci 2016;23:508-16. [Crossref] [PubMed]
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9. [Crossref] [PubMed]
- Sapir E, Tao Y, Schipper MJ, et al. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2018;100:122-30. [Crossref] [PubMed]
- Méndez Romero A, van der Holt B, Willemssen FEJA, et al. Transarterial Chemoembolization With Drug-Eluting Beads Versus Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Outcomes From a Multicenter, Randomized, Phase 2 Trial (the TRENDY Trial). Int J Radiat Oncol Biol Phys 2023;117:45-52. [Crossref] [PubMed]
- Bush DA, Volk M, Smith JC, et al. Proton beam radiotherapy versus transarterial chemoembolization for hepatocellular carcinoma: Results of a randomized clinical trial. Cancer 2023;129:3554-63. [Crossref] [PubMed]
- Comito T, Loi M, Franzese C, et al. Stereotactic Radiotherapy after Incomplete Transarterial (Chemo-) Embolization (TAE\TACE) versus Exclusive TAE or TACE for Treatment of Inoperable HCC: A Phase III Trial (NCT02323360). Curr Oncol 2022;29:8802-13. [Crossref] [PubMed]
- Dawson LA, Winter KA, Knox JJ, et al. NRG/RTOG 1112: Randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC). J Clin Oncol 2023;41:489. [Crossref]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Rizzo A, Cusmai A, Gadaleta-Caldarola G, et al. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol 2022;16:333-9. [Crossref] [PubMed]
- Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs 2022;31:361-9. [Crossref] [PubMed]


