Medication related nausea and vomiting in palliative medicine
Abstract: There are multiple potential states and/or symptoms that may occur in the palliative care population including: pain, nausea/vomiting, fatigue, anorexia, dyspnea, hiccups, cough, constipation, abdominal cramps/bloating, diarrhea, pruritis, depression/anxiety, dysphagia and sleep disturbances. Some of this may be the direct result of medications or drug-drug interactions from agents prescribed to treat the medical conditions that the patient has. Medication-related nausea and vomiting (MRNV) is a significant problem in palliative medicine that is reasonably common likely due to the multiple medications that these patients are often taking.
Key words: Medication-related nausea; vomiting; palliative
Introduction
Nausea is one of the most commonly reported side effects of medications. Clinical experience strongly suggests that both nausea and vomiting may occur with almost any medication in an individual patient. Indeed, nausea is listed as an adverse effect in FDA approved prescribing information (approved “package insert”) for most medications. Medication related nausea and vomiting frequently interferes with successful drug therapy as well as patient quality of life. For the purposes of this review, medication related nausea and vomiting (MRNV) is defined as the development of nausea, vomiting, or both which occurs secondary to the use of a medication. Retching may accompany nausea and be a precursor to vomiting. Concurrent gastrointestinal symptoms of dyspepsia (indigestion) or symptoms of acid reflux may also occur with MRNV, but are not the focus of this review. Symptoms of MRNV may occur during initiation, continuation or discontinuation (due to withdrawal syndromes) of medications, and may occur continuously or intermittently. Typically, MRNV is temporally associated with drug administration (e.g., symptoms occur with, or are shortly after drug ingestion, then diminish). Symptom severity generally varies over time, and commonly decreases with continued use of a causative medication. Medication-related vomiting is less common than nausea, and may be dose related, with nausea occurring at lower doses, progressing to both nausea and vomiting at higher therapeutic, or toxic doses.
The exact mechanisms by which most medications produce nausea and/or vomiting are unknown. It is likely that drugs produce nausea and vomiting through direct and indirect actions, with more than one mechanism of action for some agents. Not surprisingly, as nausea and vomiting is thought to be a response to possibly noxious substances, medications may be perceived by the body’s defense mechanisms as potentially toxic. Additionally, nausea and vomiting may signal a drug-related adverse effect or disease process involving the gastrointestinal or central nervous systems that secondarily results in stimulation of the pathways contributing to nausea and vomiting.
Given the frequency of MRNV, and potential of this side effect to interfere with therapy, successful management is necessary to attain optimal drug therapy outcomes. Prevention and management of medication related nausea and vomiting is highly dependent upon the clinical situation and medication(s) involved, however some general strategies can be applied to many agents. Consensus recommendations are available for management of two of the commonly encountered types of MRNV- chemotherapy induced nausea and vomiting (CINV) and post operative nausea and vomiting (PONV).
Medication related nausea and vomiting in palliative medicine is crucially important. The palliative all case population may be especially at risk for MRNV because they generally have advanced illness, advanced age, reduced renal and/or hepatic function and thus, reduced clearance; low proteins for drug binding, and multiple significant co-morbidities. Furthermore, the palliative care population tends to be on multiple medications and thus, tends to be at risk for multiple-drug drug-interaction.
Clark and colleagues studied all people referred to a community-based palliative care service over a period of 6.3 years had their bowel problem scores reported, using a numerical rating score at every clinical encounter until their death, at four discrete time points, namely, 90, 60, 30, and seven days before death (1). 3,248 (42.4%) people had disturbed bowel scores, at the time of referral; 548 to the Palliative Care Service, and (7.2%) described these as severe. Only 1,020 (13.1%) people never described disturbed bowel function over their time in palliative care. At each time point, approximately one-third were experiencing disturbed bowel function, with proportionally greater numbers of people experiencing more significant problems as death approached. Associations between bowel problem score and appetite problems, nausea, breathing problems, fatigue, and pain were explored. Although weak, there were statistically significant associations between all symptoms and bowel problem scores except for breathing problems (1). There were 22.06% experiencing nausea of any severity 90 days before death, 13.1% experiencing nausea of any severity 60 days before death, 24.8% experiencing nausea of any severity 30 days before death, and 24.07% of patients experiencing nausea of any sensitivity seven days before death (1).
Incidence of medication related nausea and vomiting
The incidence of MRNV varies widely based on the specific medication therapy involved, patient characteristics, and possibly the indication for use and treatment environment. Nausea is a common adverse drug event while emesis occurs much less frequently. Medication dose, dosage form, administration route, the rate and timing of administration and duration of use are known to influence occurrence of MRNV. Taking medication in a specific manner (such as with food) often reduces the frequency and severity of nausea. Certain medication classes are associated with high rates of MRNV, and incidence often varies between medications within the same class. Commonly, MRNV occurs early in drug therapy and wanes with continued use. Concurrent use of multiple medications, particularly if ingested at the same time, often increases MRNV. Additionally, measures of nausea used in trials are inconsistent, thus the reported frequency of MRNV will vary widely even for the same medication in the same treatment population.
The incidence of nausea and or vomiting has been reported to be as high as 70% for some drug therapies such as certain chemotherapeutics. Nausea is reported by 46% of HIV patients taking antiretrovirals. Some commonly chronically used medications such as opioid analgesics, glucose like protein 1 agonists (GLP-1) such as exenatide, and selective serotonin reuptake inhibitors such as fluoxetine produce nausea in 20-50% of patients, and rates of 20-30% are not uncommon for many medications. Note that nausea and vomiting also occur in placebo- treated groups such that the “background” incidence rate of nausea and vomiting depends on the population studied. Even in populations without underlying reasons or increased rates of nausea or vomiting, reported rates of 2-5% are common. Generally, some MRNV is an almost expected side effect with the use of multiple medications (often more than 5-10) agents so commonly used today.
Mechanisms of medication related nausea and vomiting
Despite the fact that nausea and/or vomiting (N/V) are commonly side effects, of many pharmacotherapies, the specific mechanisms involved in MRNV for most drugs are not fully understood. It is likely there are numerous direct and indirect mechanisms by which drugs produce N/V, and that medication may act through more than one of these mechanisms. The physiology of vomiting is fairly well described while nausea, the more physiologically complex symptom, and more commonly encountered adverse drug effect, is somewhat less well understood. It is probable that MRNV is the result of medications acting through the main types of stimuli known to induce nausea and vomiting from any cause:
(I) Direct or indirect stimulation of abdominal vagal and abdominal splanchic nerve afferents that project to the chemoreceptor trigger zone (CTZ), nucleus tractus sloitarius (NTS) of the vagal nerve, and directly to the vomiting (or emesis) center (VC). Stimulation of vagal and splanchic nerve afferents can result from direct activation of mucosal receptors, or indirectly through other effects such as altered gastrointestinal motility, distension, and toxic damage to G.I. tissues (2). Serotonin (5HT) release from mucosal enteroendocrine cells is thought to be the primary mediator of vagal and splanchic nerve stimulation, though Substance P and cholecystokinin may also play a role. Serotonin release is enhanced by acetylcholine (M3), norepinephrine (beta), 5HT (5HT3) and histamine (H1) receptors. Activation of these pathways can also result from medication related toxicity producing pathologic effects on the visceral organs or dysmotility.
(II) Direct stimulation of the chemoreceptor trigger zone (CTZ) in the area postrema of the fourth ventricle. Drugs could also produce CTZ activation indirectly by releasing substances from the gastrointestinal tract. The CTZ contains dopamine (D2), histamine (H1), and acetylcholine (M1) neurokinin-1, mu opiate, gaba-aminobutyric acid (GABA), N-methyl d-aspartate (NMDA), and serotonin (5HT3) receptors (3).
(III) Activation of the vomiting center through the poorly understood central nervous system stimuli. The role of the central nervous system is likely predominant when MRNV is due to unpleasant medication taste or smell as well as anticipatory N/V (4).
(IV) Activation of vestibular inputs to the VC resulting in nausea and emesis. These stimuli are thought to be primarily mediated through H1 and M1 receptors.
(V) Direct effects of medications on the NTS and/or VC through activation of 5-HT3, H1 and M1 receptors or mediated through other neurotransmitters such as Substance P (5).
The pathophysiologic mechanisms of nausea are less well understood. Nausea may be a low intesity activation of the same systems as vomiting resulting in CNS perception of nausea. Understanding of the mechanisms of MRNV can assist clinicians in management of patients experencing these symptoms. Because of the many and complex aspects of MRNV prevention and treatment, individual strategies are often insufficient, requiring additional, and often multiple therapeutic approaches to manage this common side effect.
Management of medication related nausea and vomiting
Since MRNV is most likely to be more frequent and severe upon initiation of therapy, a number of steps should be taken when patients are starting a medication. Medications should be started at the lowest appropriate dose. Some medications have specific recommendations for upward titration of dosage in order to improve initial tolerance. Close patient-provider communication and patient education can be critical during this phase of therapy (6). Medications should generally be taken with food unless specifically contraindicated. Mechanisms by which this simple strategy likely reduces MRNV through diluting drug in the upper GI tract thereby reducing local drug concentrations, improving motility, and reducing tthe rate of absorption (although for aa number of drugs food increases rate and amount absorbed), thus decreasing rate of rise and peak blood drug concentrations. Care providers should always refer to appropriate drug information resources for guidance on administration on medications. Some medication regimens are specifically designed based on timing of medication dose in trelation to food and even types of food and such recommendations should be followed.
When patients experience significant N/V when using medications, a systematic investigation into causes should be undertaken. Possible non-medication related causes and contributors should be evaluated. The relationship of symptoms to initiation of drug therapy, and to dose administration method and times, should be determined. Factors reducing or increasing the patients symptoms should be sought (7). A comprehensive review of the patient’s current medications and use of alternative medications and dietary supplements may provide useful information. Dietary habits that may exacerbate MRNV should be considered. Changing the type of food eaten (e.g., using bland foods), size and frequency of meals when taking a medication may also improve tolerance. Using enteric coated and controlled release dosage forms may aalso reduce MRNV by reducing local drug concentrations in the G.I tract or controlling the site of dosage form dissolution. Other recommendations include eating high-carbohydrate foods or drinking ginger ale, herbal or ice tea, and getting fresh cool air when feeling nauseous (8).
When taking multiple medications, tolerance may be improved by seperating the time of dose administration (when clinically appropriate) to reduce the numder of medications taken at one time. Reducing the dose frequency (e.g., taking smaller doses more often) or use controlled release dosage forms, both which reduce local GI drug conentrations and the rate of rise and peak blood drug levels. If medications are associated with dizzines or appear to produce N/V through vestibular stimulation, administration prior to sleep may be helpful. Giving in the evening or at bedtime may also improve tolerance as side effects occurring with increasing drug levels or peak blood levels will then occur while the patient is sleeping. Advise the patient that they can “experiment” to determine when and how medications are best tolerated, given this does not interfere with therapeutic efficacy.
If intolerance to the medication is still unacceptable, dose adjustment, change in dosage form or switching to alternative agent within or outside the class of medication not being tolerated. should be considered. Patients often report dramatically different tolerance to medications within the same therapeutic class and rarely even different manufacturers.. If available, feasible, and clinically appropriate, changing the route of administration (e.g., transdermal patch instead of oral) may reduce MRNV. Review the patient’s entire medication profile to determine if other medications can be contributing to the N/V, and if such therapies should be modified or discontinued.
Failing the above strategies, pharmacologic treatments may be effective in controlling MRNV. Obviously, use of any agent to treat MRNV brings further risk of adverse events.Anti-emetics are available with a number of mechanisms of action, and some are thought to work through more than one mechanism. Antiemetic agents with dopamine1 and 5HT3 blocking properties are often effective when MRNV is due to effects on the GI tract or CTZ, however antihistamines and anticholinergics may also work. When MRNV is due to GI dysmotility, a prokinetic agent as metoclopramide may be effective when other agents are not. Both dopamine blocking agents and metoclopramide carry risks for tardive dyskinesia when used long term and alternative therapy should be sought when ongoing therapy is needed. Of note is that small doses of anti-emetics are often as effective as higher doses such that antiemetic dose minimization is an appropriate approach to reducing side effects. When MRNV is due to GI dysmotility, a prokinetic agent as metoclopramide may be effective when other agents are not. Both dopamine blocking agents and metoclopramide carry risks for tardive dyskinesia when used long term. When MRNV is accompanied by vertigo or exacerbated by movement, using agents that suppress signals from the vestibular apparatus such as antihistamines and anticholinergics may be useful. Corticosteroids may be effective in MRNV due to a number of drugs (see CINV and PONV sections below), and neurokinin-1 inhhibitor is be effective in CINV and PONV. Benzodiazepine are often useful for reducing anticipatory N/V to drugs. Since MRNV may be caused by multiple mechanisms, or a patient is taking multiple medications, more than one pharmacologic agent may be needed.
Medications that commonly cause nausea and vomiting
Cancer chemotherapy
Almost all medications can cause nausea and vomiting, perhaps the most well-known cause is chemotherapy agents. There are three classifications of chemotherapy induced nausea and omitting (CINV). Acute CINV occurs within the first 24 hours after the completion of the chemotherapy infusion. Delayed CINV typically begins at least 24 hours after and can last up to several days after following the chemotherapy infusion completion. Anticipatory CINV usually occurs within 12 hours prior to treatment administration. Anticipatory CINV is usually due to a previous episode of uncontrolled vomiting with previous chemotherapy administrations (9).
The pathophysiology of CINV is not entirely understood. The two pathways though to be involved in CINV are the CTZ and the gastrointestinal (GI) tract. The CTZ is a highly vascular and is directly exposed to the chemotherapy agents in the blood and in the cerebral spinal fluid. When the enterochromoffin cells, most rapidly dividing cells in the GI tract, are damaged by the chemotherapeutic agent, serotonin is released. Serotonin binds to the vagal afferent receptors that stimulate emesis through CTZ or directly through the vomiting center.
Although the incidence of nausea correlates with the incidence of vomiting; vomiting generally occurs less frequently. There are several guidelines that exist that clearly define the prevention and treatment of chemotherapy induced nausea and vomiting. The American Society of Clinical Oncology (ASCO) (10,11) and the National Comprehensive Cancer Network (NCCN) (12) are two commonly referred to guidelines. There are slight differences between the guidelines.
There are several risk factors that make patients more predisposed to development of CINV. Patient factors include age since younger patients are more likely to experience CINV and female gender. Treatment related factors include the chemotherapy emetogenicity and the chemotherapy dose. Table 1 lists the emetogenicity of several chemotherapy agents.
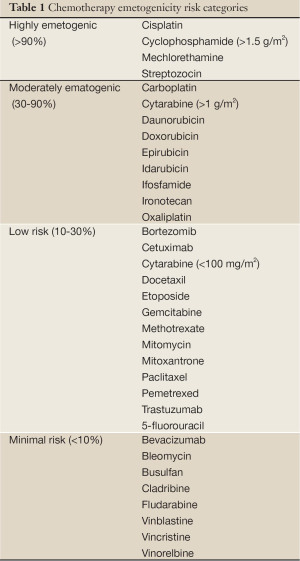
Full table
Prevention of chemotherapy induced nausea and vomiting depends on the chemotherapy regimen that the patient is receiving. Prophylactic antiemetics are selected based on the classification of CINV and the emetogenicity of the chemotherapeutic agent (Table 2). Dexamethasone is a well established antiemetic for patients who receive highly emetogenic agents. More detailed explanations can be found in the NCCN and ASCO guidelines.
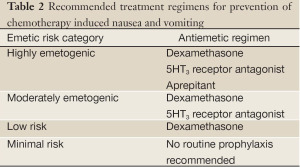
Full table
For patients who are experiencing anticipatory CINV, lorazepam or alprazolam can be recommended. These agents have both amnesic and anti-anxiety properties that would be beneficial.
Medications used in peri-operative care
Post-operative nausea and vomiting (PONV) continues to be an unfavorable consequence of surgery and anesthesia. Nausea and vomiting should be considered individual entities and therefore should be assessed independently. The frequency of PONV ranges from 10-80%. In the ambulatory care setting, PONV rates range from 30-50%.
The exact etiology of PONV is unknown. The major receptors involved in the development of PONV are dopaminergic, cholinergic, histaminergic, serotonergic and neurokinin (NK-1). Stimulation of the vestibular system during surgery, increased vestibular sensitivity due to opiod administration and movement following surgery can lead to PONV. Using morphine to manage post-operative pain has been associated with an increase the frequency of PONV. Opioids on their own can cause nausea and vomiting by direct stimulation of the CTZ, delaying gastric emptying, reducing gastrointestinal motility and sensitizing the vestibular system to motion.
It is suggested that there are a number of patient-specific, surgical and anesthetic factors that can lead to the development of PONV (Tables 3,4). Nitrous oxide is known to cause N/V when administered as the sole anesthetic agent. Other predictors include the use of peri-operative opioids and dehydration prior to surgery. Aggressive hydration prior to surgery has been shown to reduce the risk of post-operative nausea.
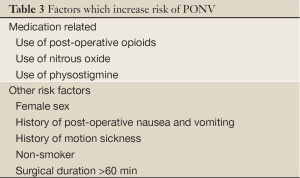
Full table

Full table
Female sex , non-smoking status and general anesthesia are factors which of both post-operative nausea and post-operative vomiting while history of migraine and the type of surgery are more predictive for post-operative nausea alone. Inhaled anesthetic agents are the strongest risk factor in the development of post-operative vomiting.
PONV frequency is highly dependent on the number of risk factors that a patient has. Patient with only one risk factor have lower tendency to develop PONV, 20-30%; conversely patients with two or more risk factors have the highest risk of developing PONV, 60-80%.
Patients should be assessed prior to surgery for their risk of PONV. In patients who are high risk, administration of a prophylactic antiemetic should be considered. The agent should block at least one for the receptors thought to be involved PONV (dopaminergic, cholinergic, histaminergic, serotonergic and neurokinin). None of the available agents are entirely effective for the prevention of PONV, especially in patients who are considered high risk (13). Thus, it is recommended that clinicians administer combinations of antiemetic treatments from different drug classes (with different mechanisms of action) (14,15).
Opioid analgesics
Opioid analgesics are a well recognized cause of N/V both during acute and chronic use. Nausea and vomiting is often cited by patients as an “allergy” to opioids, and a good medication history to explore the patients symptoms should be performed. Opioids contibute considerably to the problem of PONV and N/V in cancer patients. (see sections on PONV and CINV above). Nausea and vomiting is a major reason for treatment discontinuation in long-term opioid use. In acute use of opioids post operatively, nausea is reported in up to 70% of patients and and vomiting in up to 40%. Chronic opioid use is associated with nausea in 10-40% of patients, with vomiting occurring in 7-12% (16). In general the incidence and severity of N/V decreases with continued use, but a significant minority of patients will have continuing symptoms. Orally administered opioids appear to produce more N/V than parenterally administered drug. Transdermal fentanyl generally elicits N/V at the same rate as other chronic opioids, but may be better tolerated in an individual patient (16).
The opioids produce N/V through activation of opioid receptors (mu, delta and kappa).in the central nervous system and periphery (17). Peripherally, opioids alter gut motility (mu and kappa receptors) (18,19), direct activation of the CTZ (mu and delta receptors) and activation or sensitization of the vestibular apparatus (mu and possibly delta and kappa receptors). Cortical inputs may also play a role in opioid nausea and vomiting. Note that at higher doses, opioids may act as an anti-emetic. The role of any specific mechanism possibly varies by patient, specific agent, clinical situation, route of administration, and duration of use.
All opioids may be associated with nausea and vomiting, and controlled trials typically demonstrate a similar frequency for compared agents. There is wide interpatient variability in tolerance of opioids with patients experiencing significant N/V with one agent, but tolerating other opioids. This variability most likely results in differences in drug action at opioid receptors and interpatient variability in drug metabolism and opioid receptor expression. Some clinicians consider codeine and morphine to be more likely to produce N/V. However controlled trials have note born out this “clinical lore”. Chang et al. found morphine and hydromorphone to be equally effective and to produce N/V at the same rates when given as a bolus dose in emergency room patients (20) and Hong et al. found the two agents equivalent when administered as patient controlled analgesia (21). The agonist antagonist opioids (e.g., butorphanol and nalbuphine) may produce less N/V, but have limited clinical utility in many settings.
The important point is that reponse varies from drug to drug and patient to patient. Tapentadol, a new “non-opioid” analgesic agent with mu opioid agonist effects as well as norepinephrine reuptake blocking activity, produces less nausea and vomiting than pure mu agonists, however with rates of nausea as high as 50% (compared to 70% for oxycodone) and vomiting frequency of 17% (compared to 40% for oxycodone) during post operative use, MRNV remains highly problematic (22).
Management of opioid related N/V involves both general and targeted strategies. General strategies include modification of drug, dose, route, dosage form or dose regimen. Providing an appropriate bowel regimen to avoid constipation may reduce N/V. Non-pharmacologic strategies include use of relaxation techniques, dietary alteration (avoiding spicy and salty foods or heavy sweets) and environmental changes (cool fresh air, lower lights, limit moving objects) (23). No pharmacologic class has been shown to be superior in the treatment of opioid N/V (24). Dopamine blockers, 5HT3 blockers, antihistamines, anticholinergics and steroids all appear to be equally effective (or equally ineffective) as single agents (25). Dopamine antagonists, and 5HT3 inhibitors are often effective when opioid N/V is due to direct and indirect CTZ stimultion. When opioid N/V is associated with early satiety, bloating, constipation or early post-meal vomiting, treatment with metoclopramide to increase gut motility may help somewhat in conjunction with other strategies. When N/V occurs with vertigo, use of an antihistamine or anticholinergic should be considered. Combination therapy having antiemetics with different mechanisms of action is often more effective than single agent therapy. Other pharmacologic agents with evidence of effect on opioid N/V include using low dose opioid antagonists (naloxone, naltrexone) and agonist-antagonist opiates. Due to the incomplete blood- brain barrier of the area postrema (CTZ), peripheral mu antagonists block opioid induced N/V without reducing pain control. However the clinical use of such agents (e.g., methylnaltrexone) has not been fully evaluated for the treatment of opoioid for the treatment of opioid-induced nausea (26). Benzodiazeines may be efective for anticipatory N/V associated with opioids. Corticosteroids and the neurokinin-1 antagonists are effective in control of PONV. However there is no data available demonstrating effectiveness of neurokinin-1 antagonists when opioid use alone is the cause of N/V. The atypical antipsychotic agent risperidone may be effective when other agents are not. Given the multiple mechanisms of opioid N/V, more than one therapy may be required to manage same patients.
Antiretrovirals
Nausea and vomiting is an extremely common and bothersome symptom reported by HIV patients. Nausea in HIV is often caused or exacerbated by antiretrovirals, reducing quality of life and adherence to therapy (27). Note that HIV patient often concurrently receive other medications that produce N/V frequenly including opioids, SSRI and antimicrobials.
In patients treated with antiretrovirals or occupational and non-occupational HIV exposure prohylaxis (zidovudine, lamivudine together, with indinavir, nelfinavir or tenofovir), 42-53% experienced nausea and 14-16% vomited (28). Combination antiretroviral therapy given to treatment naive asymptomatic HIV postive patients treated with indinavir/zidovudine/lamivudine 69% reported nausea ans\d 42% vomiting (29). In general, nausea occurs in 25% of patients taking initial antiretroviral therapies, is the most common cause of drug discontinuation and is correlated with treatment failure (30). Patients with advanced disease requiring complex regimens are more likely to experience N/V (31). Overall, 25% of highly active antiretroviral therapy discontinuations are a result of nausea and vomiting (32). Symptoms are typically worse upon therapy initiation, however ongoing nausea and vomiting are not uncommon. The frequency of N/V varies between available antiretroviral regimens (33).
Antiretroviral agents most commonly associated with nausea and vomiting are the non-nuceoside reverse transcriptase inhibitors and protease inhibitors, in particular nevirapine, ritonavir, amprenavir, abacavir and efavirenz. Nausea and vomiting from antiretrovirals likely results from both effects on the gastrointestinal tract and through activation of the CTZ. In patients with previous severe nausea and vomiting from antiretrovirals, anticipatory nausea and vomiting may possibly contribute. Management of antiretroviral induced nausea and vomiting includes administration with food or meals, changing foods eaten prior to taking doses, taking smaller but more frequent meals, relaxation techniques, altering timing of doses and switching of medications and dosage forms. A number of suggestions for non-pharmacologic management have been recommended (Table 5) (34). Providers need to be aware of recommendations for administration of each agent the patient is taking and to provide effective education on proper use.
Full table
Medications useful in antiretrovirals include the commonly used anti-emetics, plus dronabinol and medical marijauna. Because of the frequency of MRNV with non-antiretroviral medications used in HIV patients, review and possible adjustment of other pharmacologic agents taken by the patient should be undertaken. Again, the complexity of HIV patients may require the use of multiple strategies aimed at reducing N/V to an acceptable level for the individual patient.
Other medications
Most medications are associated with nausea and less commonly, vomiting in a small minority of patients. However, MRNV may become a significant issue with some therapies. Table 6 lists medications which are associated with N/V in 10% or more of patients compiled from standard drug information resources, primary and secondary medical literature and the FDA approved prescribing information. Factors that will influence reported rates of N/V include “background” rates of N/V in the treated population, asessment tools and measurment of MRNV, timing of MRNV assesment, and concurrent therapies. Because of the variablliity of study populations and methods of adverse event detection and assessment, comparing the frequency of N/V associated with different therapeutic options using available drug information resources is of limited value unless very large differences in reported rates exist.
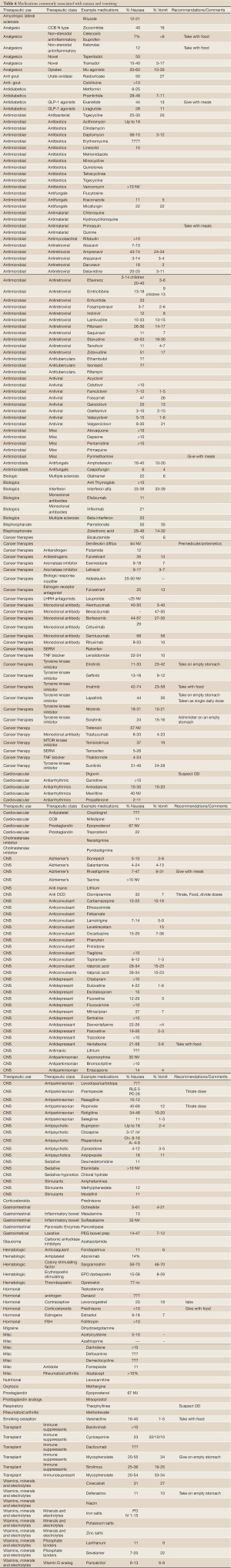
Full table
Summary
Medication-related nausea and vomiting (MRNV) is a significant clinical issue in the palliative care population likely due to the multiple medications that these patients are often taking. A greater appreciation of the etiologies contributing to; and associated with MRNV may help lead to optimal outcomes for the palliative care population.
Acknowledgements
The author would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript.
Disclosure: The author has no disclosures and has not published or submitted this manuscript elsewhere.
References
- Clark K, Smith JM, Currow DC. The prevalence of bowel problems reported in a palliative care population. J Pain Symptom Manage 2012;43:993-1000.
- Hasler WL, Chey WD. Nausea and vomiting. Gastroenterology 2003;125:1860-7.
- Sanger GJ, Andrews PLR. Treatment of nausea and vomiting: Gaps in our knowledge. Auton Neurosci 2006;129;3-16.
- Rhodes VA, McDaniel RW. Nausea, vomiting, and retching: Complex problems in palliative care. CA Cancer J Clin 2001;51:232-48.
- Quigley EM, Hasler WL, Parman HP. AGA technical review on nausea and vomiting. Gastroenterology 2001;120:263-86.
- Garrett K, Tsuruta K, Walker S, et al. Managing nausea and vomiting: Current strategies. Crit Care Nurs 2003;23:31-50; quiz 51.
- Metz A, Hebbard G. Nausea and vomiting in adults. A diagnostic approach. Aust Fam Physician 2007;36:688-92.
- Hasler WL. Approach to the patient with Nausea and Vomiting. In: Yamada T. eds. Principles of Clinical Gastroenterology. Oxford, UK: Wiley-Blackwell, 2009:205-27.
- Hesketh PJ. Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2008;358:2482-94.
- Basch E, Prestrud AA, Hesketh PJ, et al. American Society of Clinical Oncology. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189-98.
- Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncolgy Guidelines for Antiemetics in Oncology: Update 2006. J Clin Oncol 2006;24:5341-2.
- Ettinger DS, Armstrong DK, Barbour S, et al. National Comprehensive Cancer Network. Antiemesis. J Natl Compr Canc Netw 2012;10:456-85.
- Golembiewski J, Chernin E, Chopra T. Prevention and treatment of postoperative nausea and vomiting. Am J Health-Syst Pharm 2005;62:1247-60.
- Kloth DD. New pharmacologic findings for the treatment of PONV and PDNV. Am J Health-Syst Pharm 2009;66:S11-S18.
- Habib AS, Gan TJ. Evidence-based management of postoperative nausea and vomiting: a review. Can J Anaesth 2004;51:326-41.
- McNicol E, Horowics-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic non-cancer pain: a systematic review. J Pain 2003;4:231-56.
- Porreca F, Ossipov M. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications and management options. Pain Medicine 2009;10:654-62.
- Wood JD, Galligani JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil 2004;16:17-28.
- De Schepper HU, Cremonini, Camilleri M. Opioids and the gut: pharmacology and current clinical experience. Neurogastroenterol Motil 2004;16:383-94.
- Chang AK, Bijur PE, Meyer RH, et al. Safety and efficacy of hydromorphone as an analgesic alternative to morphine in acute pain: A randomized clinical trial. Ann Emerg Med 2006;48:164-72.
- Hong D, Flood P, Diaz G. The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg 2008;107:1384-9.
- Daniels SE, Upmalis D, Okamoto A, et al. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin 2009;25:765-76.
- Swegle JM, Logemann C. Management of common opioid -induced adverse effects. Am Fam Physician 2006;74:1347-54.
- Herndon CM, Jackson KC, Hallin PA. Management of opioid induced gastrointestinal effects in patients receving palliative care. Pharmacotherapy 2002;22:240-50.
- Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effect of oral morphine: an evidence based report. J Clin Oncol 2001;19:2542-54.
- Yuan C-S. Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunctionand other opioid adverse effects. Ann Pharmacother 2007;41:984-993.
- Harding R, Molloy T, Easterbrook P, et al. Is retroviral therapy associated with symptom prevelence and burdon? Int J STD AIDS 2006;17:400-5.
- Luque A, Hulse S, Wang D, et al. Assessment of advrerse events associated with antiretroviral regimens for exposure prophylaxis for ocupational and nonoccupational exposures to prevent transmission of human immunodeficiency virus. Inf Contol Hosp Epidemiol 2007;28:695-701.
- McMahon DK, DiNubile MJ, Meibohm AR, et al. Efficacy, safety and tolerability of long-term combination antiretroviral therapy in asymptomatic treatment-naive adults with early HIV infection. HIV Clin Trials 2007;8:269-81.
- Carr A, Amin J. Efficacy and tolerability of initial antiretroviral therapy: a systematic review. AIDS 2009;23:343-53.
- Chubineh S, McGowan J. Nausea and vomitinhg in HIV: a symptom review, Int J STD AIDS 2008;19:723-28.
- Reynolds NR, Neidig JL. Characteristics of nausea reported by HIV-infected patients initiating combination antiretroviral regimens. Clin Nurs Res 2002;11:71-88.
- Hill A, Balkin A. Risk factors for gastrointestinal adverse events in HIV ttreated and untreated patients. AIS Rev 2009;11:30-8.
- Highleyman L. Managing nausea, vomiting, and diarrhea. BETA 2002;15:29-39.


